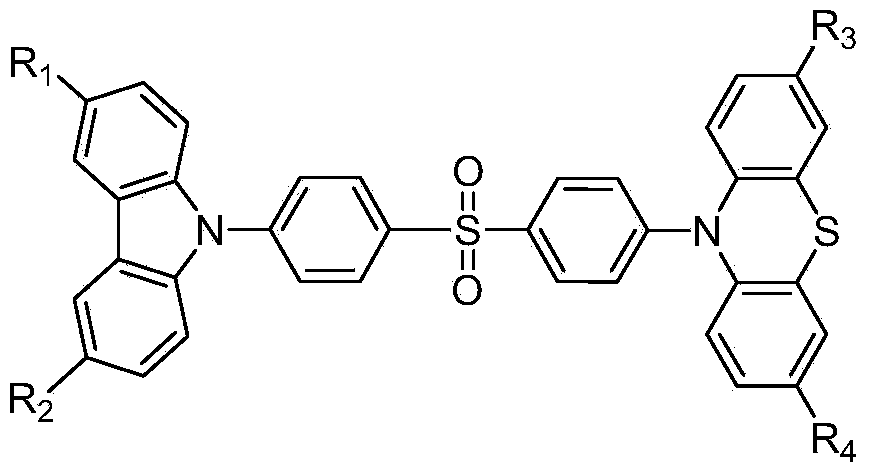
Novel piezoluminescence material with thermal activation delayed fluorescence and aggregation-induced emission properties and synthetic method and application of novel piezoluminescence material
A technology of aggregation-induced luminescence and thermal activation delay, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of low luminous efficiency of piezoluminescent materials, etc. The effect of a simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The synthesis method of the above-mentioned luminescent material is as follows: react carbazole or substituted carbazole intermediate with 4,4'-difluorodiphenyl sulfone to obtain monofluorodiphenyl sulfone with carbazolyl or substituted carbazolyl at one end, and then react with Reaction of phenothiazine or substituted phenothiazine intermediates. The described substituted carbazole intermediate and substituted phenothiazine intermediate are commercially available products, or the alkyl group is connected to carbazole and phenothiazine through Friedel-Crafts alkylation reaction, or it is formed by using Suzuki couple The joint reaction connects an aromatic group or an aromatic heterocyclic group to carbazole and phenothiazine. Specifically include the following steps:
[0019] The first step: synthesis of alkyl or aryl substituted carbazole or phenothiazine intermediates.
[0020] Utilize Friedel-Crafts alkylation reaction to prepare alkyl-substituted carbazole or phe...
Embodiment 1
[0032] (1) Add carbazole (3.34g, 0.02mol), 4,4'-difluorodiphenylsulfone (5.09g, 0.02mol) into a three-neck flask, add 100mL N,N-dimethylformamide (DMF) 1. Triethylamine (2.02g, 0.02mol), stirred and blown with argon, heated to 90°C for 24h. Extract with water and dichloromethane, dry the organic phase with anhydrous sodium sulfate, and spin dry in a rotary evaporator under vacuum to obtain a crude product. Purification is carried out by silica gel column chromatography, and a mixed solvent of n-hexane and dichloromethane in a certain volume ratio is used as an eluent. Yield 68%.
[0033]
[0034](2) Add phenothiazine (1.99g, 0.01mol), carbazole monofluorodiphenyl sulfone (4.01g, 0.01mol) into a three-neck flask, add 50mL N,N-dimethylformamide (DMF), Potassium tert-butoxide (0.96 g, 0.01 mol) was heated to 90° C. for 24 h after stirring and flowing argon. Extract with water and dichloromethane, dry the organic phase with anhydrous sodium sulfate, and spin dry in a rotary ...
Embodiment 2
[0037] Referring to the step (1) of Example 1 for the synthesis of tert-butylcarbazole-substituted monofluorodiphenyl sulfone (yield rate is 70%), and then referring to step (2) to utilize tert-butylphenothiazine to synthesize the final product, the yield rate is 75%. %, the solid fluorescence efficiency is 92%.
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com