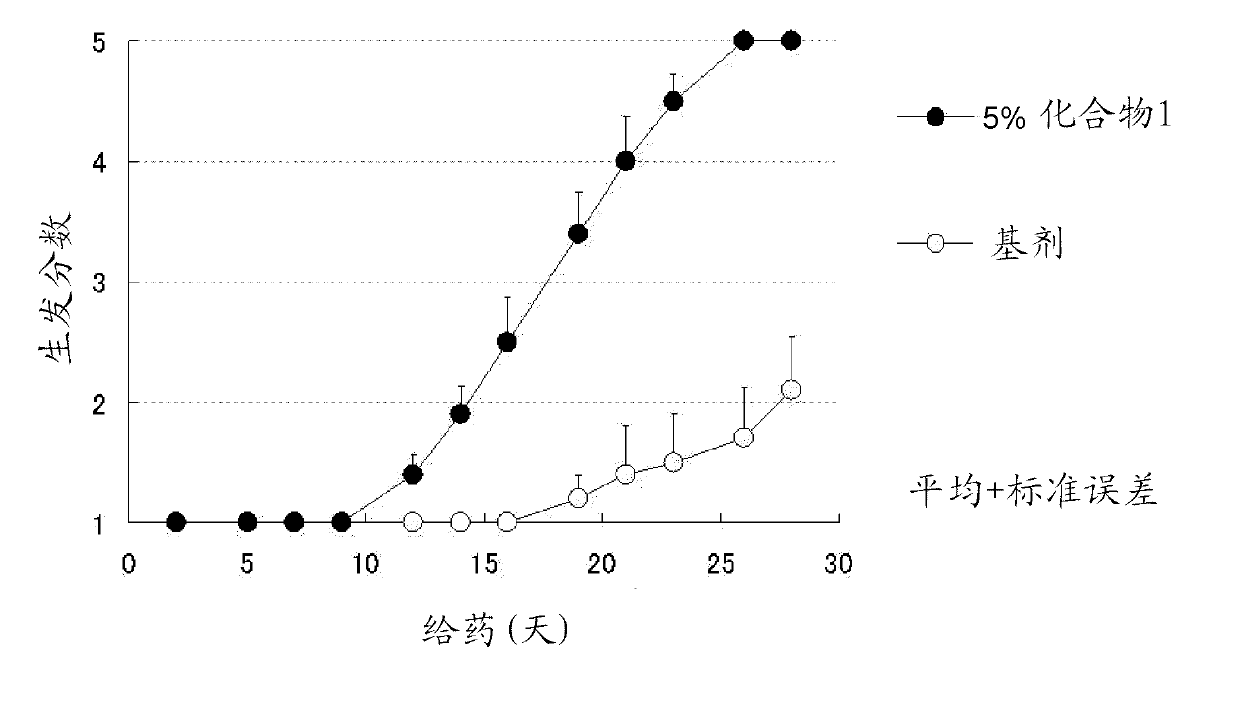
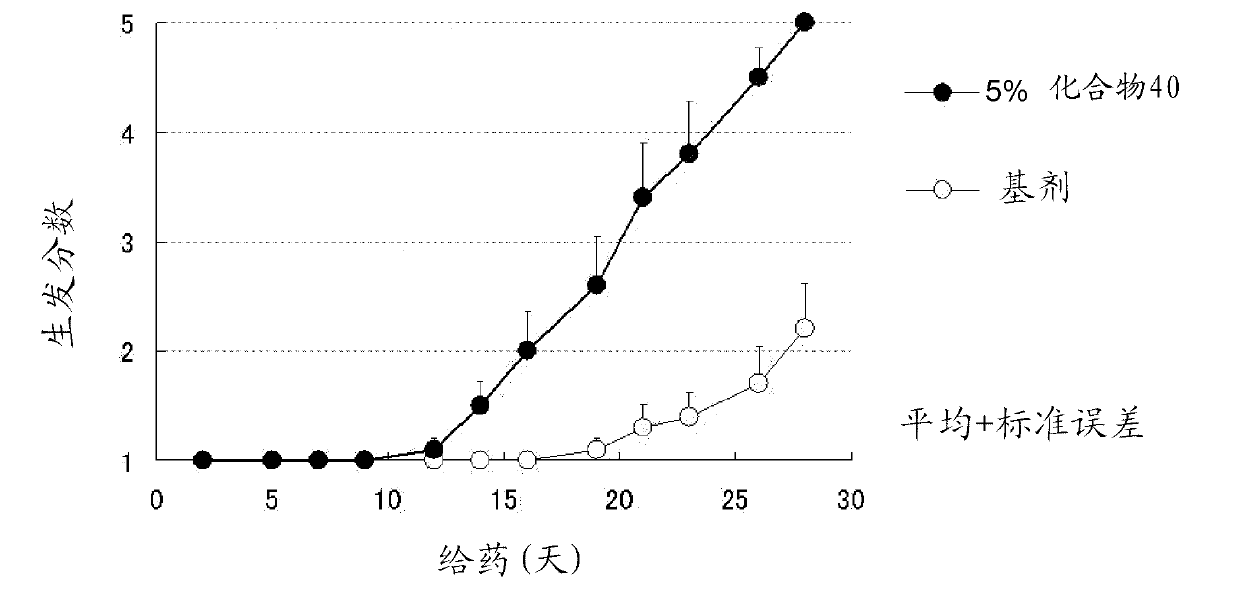
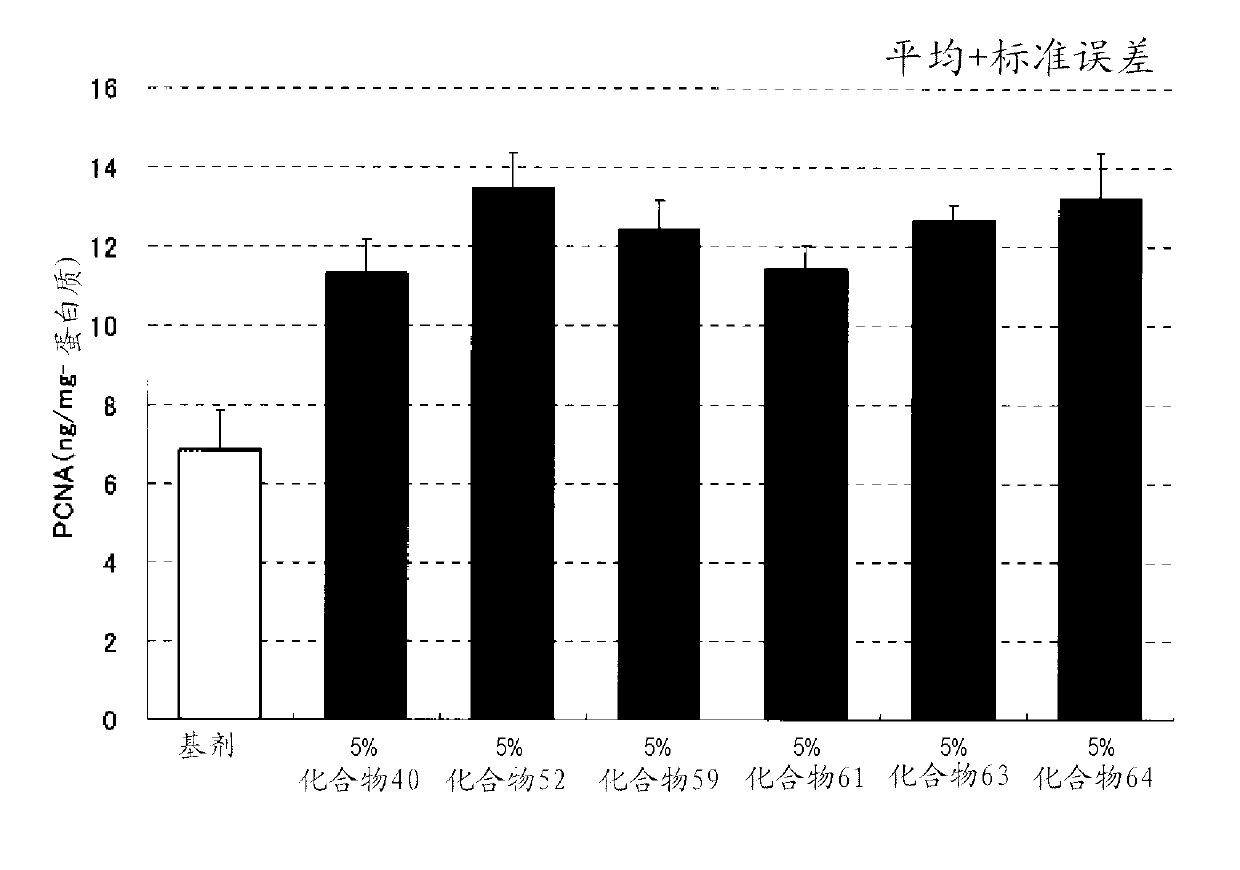
Azole derivative
一种吡咯烷、化合物的技术,应用在吡咯衍生物领域,能够解决没有公开吡咯结构、抑免蛋白FKBP12键合活性和生发促进活性的关联不清楚等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0264] [chemical formula 18]
[0265]
[0266] (S)-1-(2-(5-((3,4-dimethoxyphenoxy)methyl)isoxazol-3-yl)pyrrolidin-1-yl)-2,2-di Fluoro-2-(1-hydroxy-3,3,5,5-tetramethylcyclohexane)ethanone (compound 1)
Embodiment 1-(1)
[0268] [chemical formula 19]
[0269]
[0270] (S)-5-((3,4-dimethoxyphenoxy)methyl)-3-(pyrrolidin-2-yl)isoxazole
[0271] Under argon atmosphere, add 1,2-dimethoxy-4-(prop-2-yn-1-yloxy)benzene (81.85g) in THF (1000mL) at 60℃~-70℃ Add n-BuLi (147mL, 2.76N hexane solution) dropwise over 55 minutes, and after stirring at the same temperature for 30 minutes, add (S)-tert-butyl 2-(methoxy(methyl)carbamoyl) dropwise ) THF (600 mL) solution of pyrrolidine-1-carboxylate (100.00 g), heated up to room temperature over 2 hours, and stirred at 25° C. for 30 minutes. The reaction solution was added to saturated NH 4 Cl aqueous solution (3L), ice water (2L), hexane (1L) and AcOEt (1L), the organic layer was separated, washed with brine (5L), water (2L), brine (1L) successively, and dried (MgSO 4 ), filtered, concentrated and dissolved the obtained brown oil (161.24g) in EtOH (1000mL), added hydroxyamino hydrochloride (53.79g), heated to reflux for 13 hours, and stirred at room tempe...
Embodiment 1-(2)
[0274] [chemical formula 20]
[0275]
[0276] (S)-1-(2-(5-((3,4-dimethoxyphenoxy)methyl)isoxazol-3-yl)pyrrolidin-1-yl)-2,2-di Fluoro-2-(1-hydroxy-3,3,5,5-tetramethylcyclohexane)ethanone (compound 1)
[0277] 2,2-Difluoro-2-(1-hydroxy-3,3,5,5-tetramethylcyclohexane)acetic acid (87.83g) and Et 3 Ethyl chloroformate (30.8 mL) was added to a solution of N (122 mL) in THF (2000 mL), and stirred for 30 minutes. A THF (500 mL) solution of the compound (89.00 g) obtained in Example 1-(1) was added dropwise to the reaction mixture over 1 hour at room temperature, followed by stirring at room temperature for 20 hours. The reaction mixture was concentrated, AcOEt (2 L) and saturated NH 4 Cl aqueous solution (2L), after filtering the insoluble matter, the separated organic layer was washed with saturated aqueous sodium bicarbonate solution (2L), dried (MgSO 4 ), filtered, and concentrated to obtain a crude product (165.0 g). Follow the same method using 2,2-difluoro-2-(1-hydrox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com