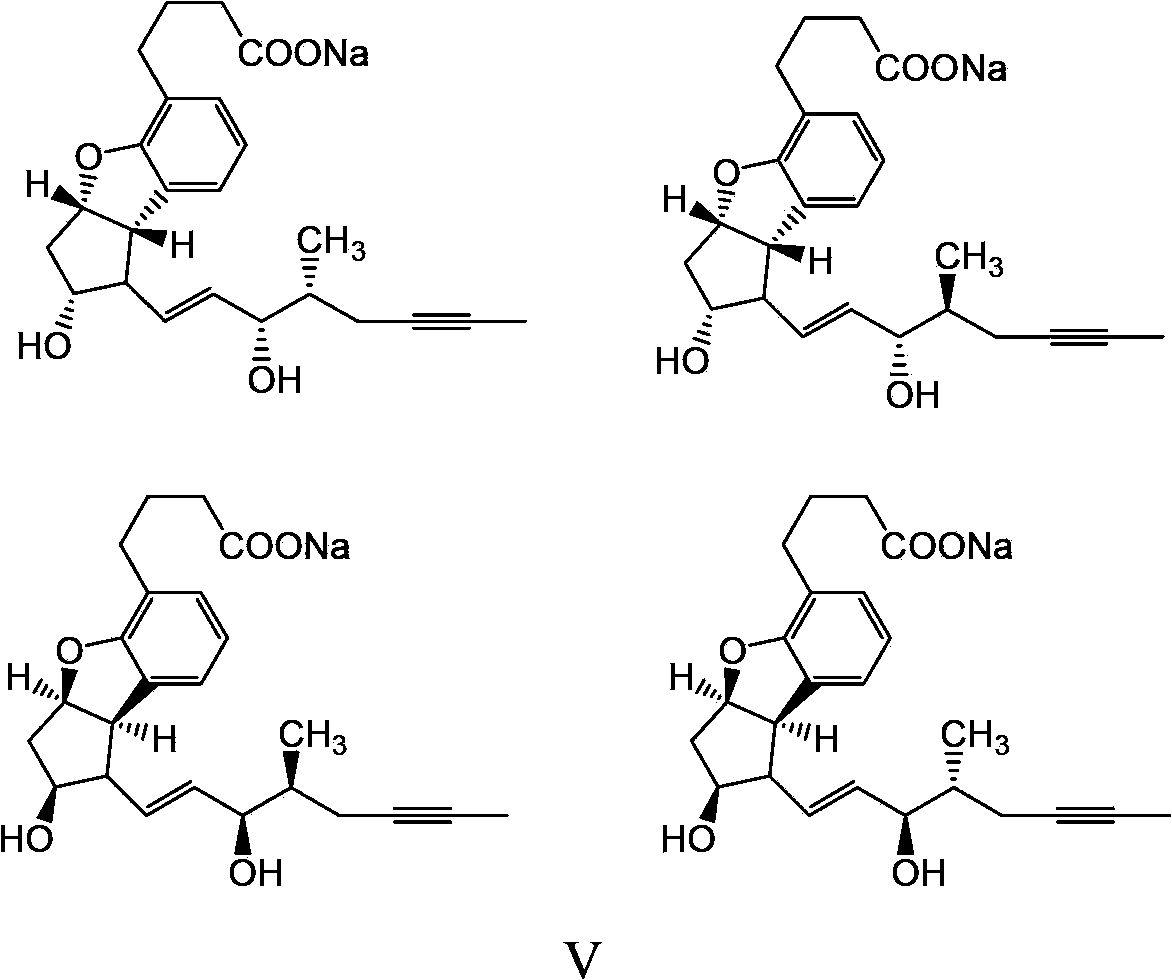
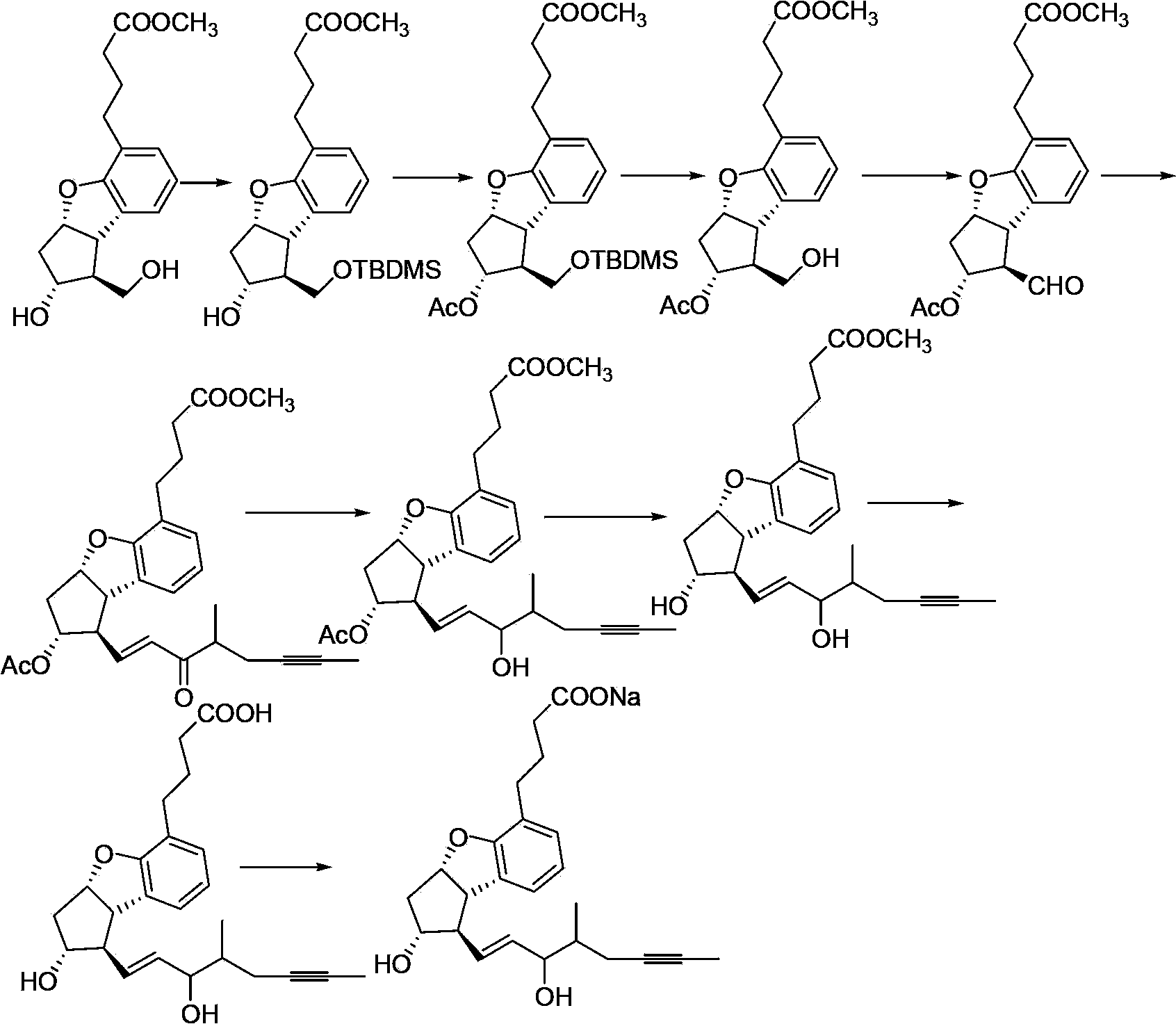
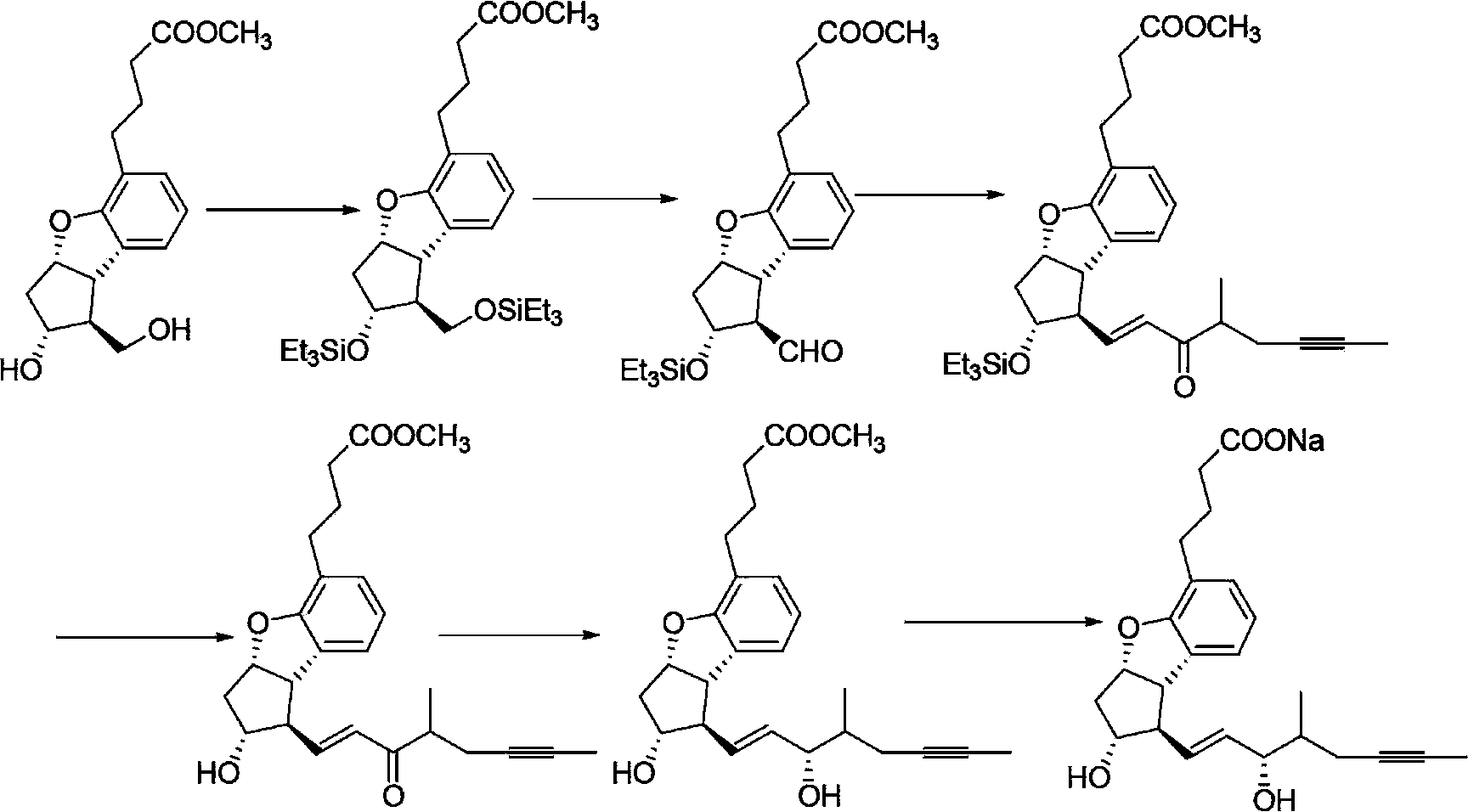
Beraprost sodium intermediates and preparation method thereof
A beraprost sodium and compound technology, applied in the field of beraprost sodium intermediates and their preparation, can solve the problems of high equipment requirements, short steps, high toxicity, etc., and achieve the avoidance of toxic reagents, mild reaction conditions, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The preparation method of formula I compound
[0077] The preparation method of the compound of formula I provided by the invention comprises the following steps:
[0078]
[0079] (a) using the compound of formula II as a raw material, protecting the hydroxyl group of the compound of formula II to generate a compound of formula I of the structure shown in formula Ia; and optionally
[0080] (b) the compound of formula Ia removes the protecting group R' to obtain the compound of formula I of the structure shown in formula Ib; and optionally
[0081] (c) the compound of formula Ib is oxidized to obtain the compound of formula I of the structure shown in formula Ic; and optionally
[0082] (d) the compound of formula Ic reacts with the phosphoric acid ester shown in formula X, obtains the compound of formula I of structure shown in formula Id,
[0083]
[0084] In the formula, Ri and Rii are independently selected from C 1-4 alkyl;
[0085] In the various formul...
Embodiment 1
[0122] The preparation of formula II compound
[0123] (where R 1is methyl, R' is tert-butyldiphenylsilyloxy)
[0124] In a 500ml four-neck bottle, 15g of the compound of formula Z (R 1 (methyl) was dissolved in 100ml of dichloromethane, 5ml of triethylamine and 0.34g of DMAP were added, under nitrogen protection, cooled to 0°C, and 50ml of dichloromethane solution containing 12.8g of tert-butyldiphenylchlorosilane was added dropwise , stirred for 2 hours, and the reaction was complete. Add 75ml of dilute citric acid aqueous solution to wash several times, wash with 300ml saturated NaCl solution, dry over anhydrous sodium sulfate, filter, and concentrate to obtain a slightly yellow liquid. After purification by column chromatography, 26.1 g was obtained, yield: 97.9%. The reaction product of this step can be put into the next step reaction without purification.
[0125] MS:567.5[M+Na] +
[0126] 1 H-NMR (400MHz, CDCl 3 ), δH: 0.75(9H,s), 1.75-2.84(11H,m), 3.52(3H,s), ...
Embodiment 2
[0128] The preparation of formula II compound
[0129] (where R 1 is ethyl, R' is tert-butyldimethylsilyloxy)
[0130] In a 500ml four-neck bottle, 15g of the compound of formula Z (R 1 is ethyl) dissolved in 100ml of dichloromethane, add 5ml of triethylamine, 0.33g of DMAP, under the protection of nitrogen, cool to 0°C, drop into 50ml of dichloromethane solution containing 11.6g of tert-butyldimethylsilyl chloride , stirred for 2 hours, and the reaction was complete. Add 75ml of dilute citric acid aqueous solution to wash several times, wash with 300ml saturated NaCl solution, dry over anhydrous sodium sulfate, filter, and concentrate to obtain a slightly yellow liquid. After purification by column chromatography, 20.2 g was obtained, yield: 99.3%. The reaction product of this step can be put into the next step reaction without purification.
[0131] MS:457.5[M+Na] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com