Preparation method of ertapenem sodium salt
A technology of ertapenem sodium salt and solvent, applied in the field of preparation of ertapenem sodium salt, can solve the problems of potential safety hazards and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation method of the ertapenem sodium salt of an embodiment comprises the following steps:
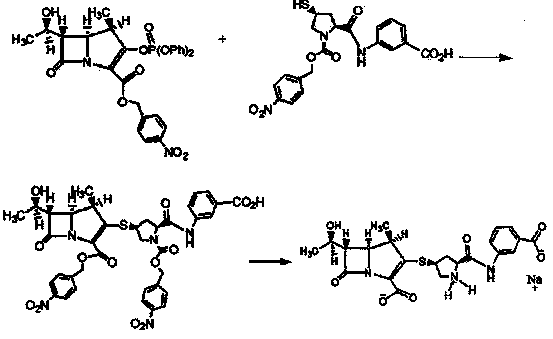
[0018] (1) Under the condition of -30°C, the penem core MAP with the protective group and the side chain of ertapenem undergo a docking reaction in the solvent tetrahydrofuran and triethylamine to generate an intermediate product; and
[0019] (2) The above intermediate product was deprotected by hydrogenation with palladium-charcoal as a catalyst and sodium bicarbonate as an alkalizing agent to obtain ertapenem sodium salt, namely [4R,5S,6S]-3-[[(3S, 5S)-5-[[(3-carboxyphenyl)amino]-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo- 1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monosodium salt, the yield was 98%. Its synthetic route is shown in the following formula:
[0020]
Embodiment 2
[0022] The preparation method of the ertapenem sodium salt of an embodiment comprises the following steps:
[0023] (1) Under the condition of -0°C, the penem core MAP with a protective group of formula I and the side chain of ertapenem of formula II were docked in the solvent diisopropylamine and acetonitrile to generate an intermediate product ;and
[0024] (2) The above intermediate product was deprotected by hydrogenation with palladium-charcoal as a catalyst and sodium bicarbonate as an alkalizing agent to obtain ertapenem sodium salt, namely [4R,5S,6S]-3-[[(3S, 5S)-5-[[(3-carboxyphenyl)amino]-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo- 1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monosodium salt, yield 98.5%.
[0025]
Embodiment 3
[0027] The preparation method of the ertapenem sodium salt of an embodiment comprises the following steps:
[0028] (1) Under the condition of 20°C, the penem mother nucleus MAP with a protective group of formula I and the side chain of ertapenem of formula II were docked in the solvent diisopropylethylamine and dichloromethane, generate intermediate products; and
[0029] (2) The above intermediate product was deprotected by hydrogenation with palladium-charcoal as a catalyst and sodium bicarbonate as an alkalizing agent to obtain ertapenem sodium salt, namely [4R,5S,6S]-3-[[(3S, 5S)-5-[[(3-carboxyphenyl)amino]-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo- 1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monosodium salt, the yield is 98.8%.
[0030] By using the above preparation method to synthesize ertapenem sodium salt, the yield is greatly improved, and the cost is reduced, and the operation steps are simplified; the required reagents are cheap and environ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com