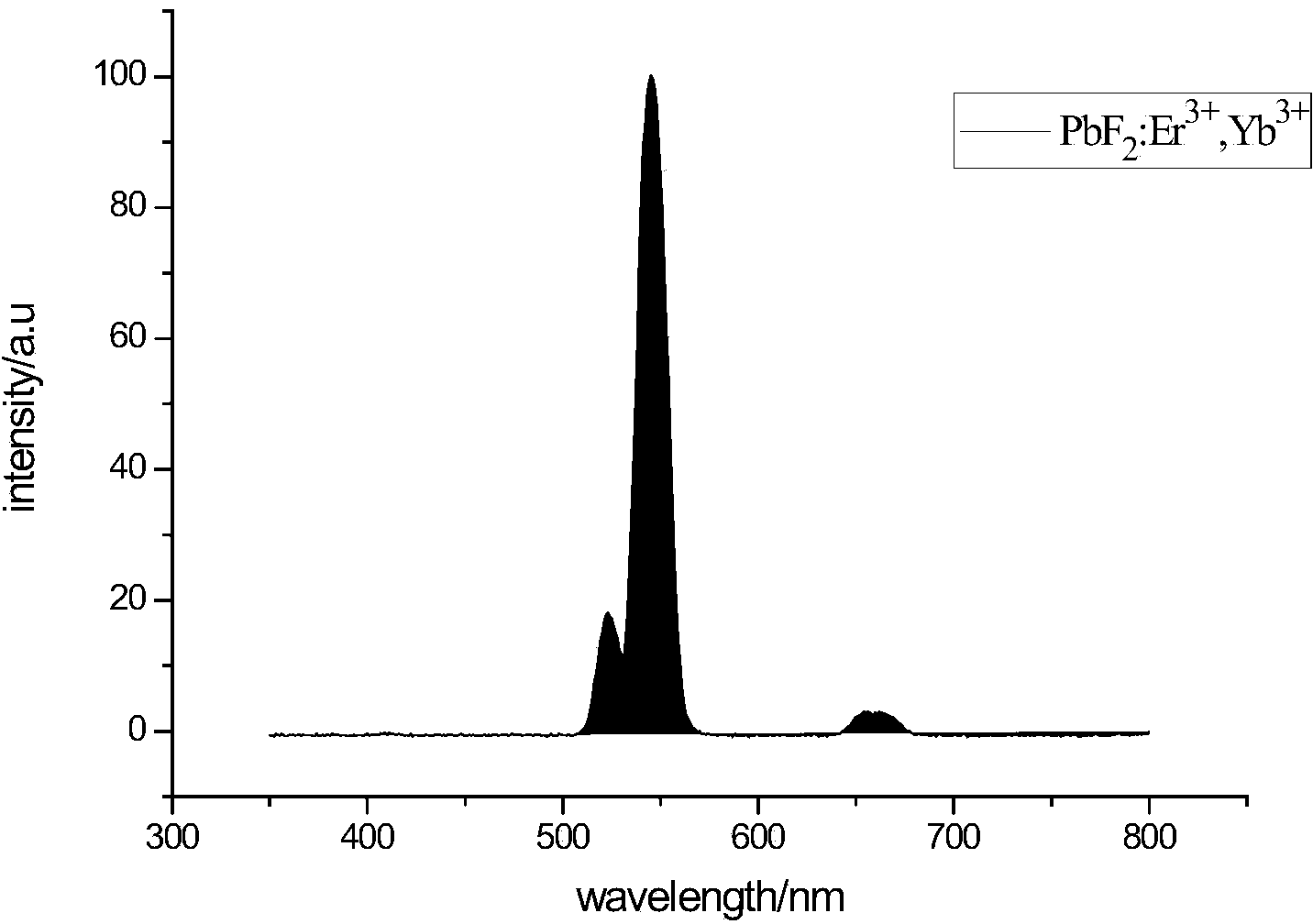
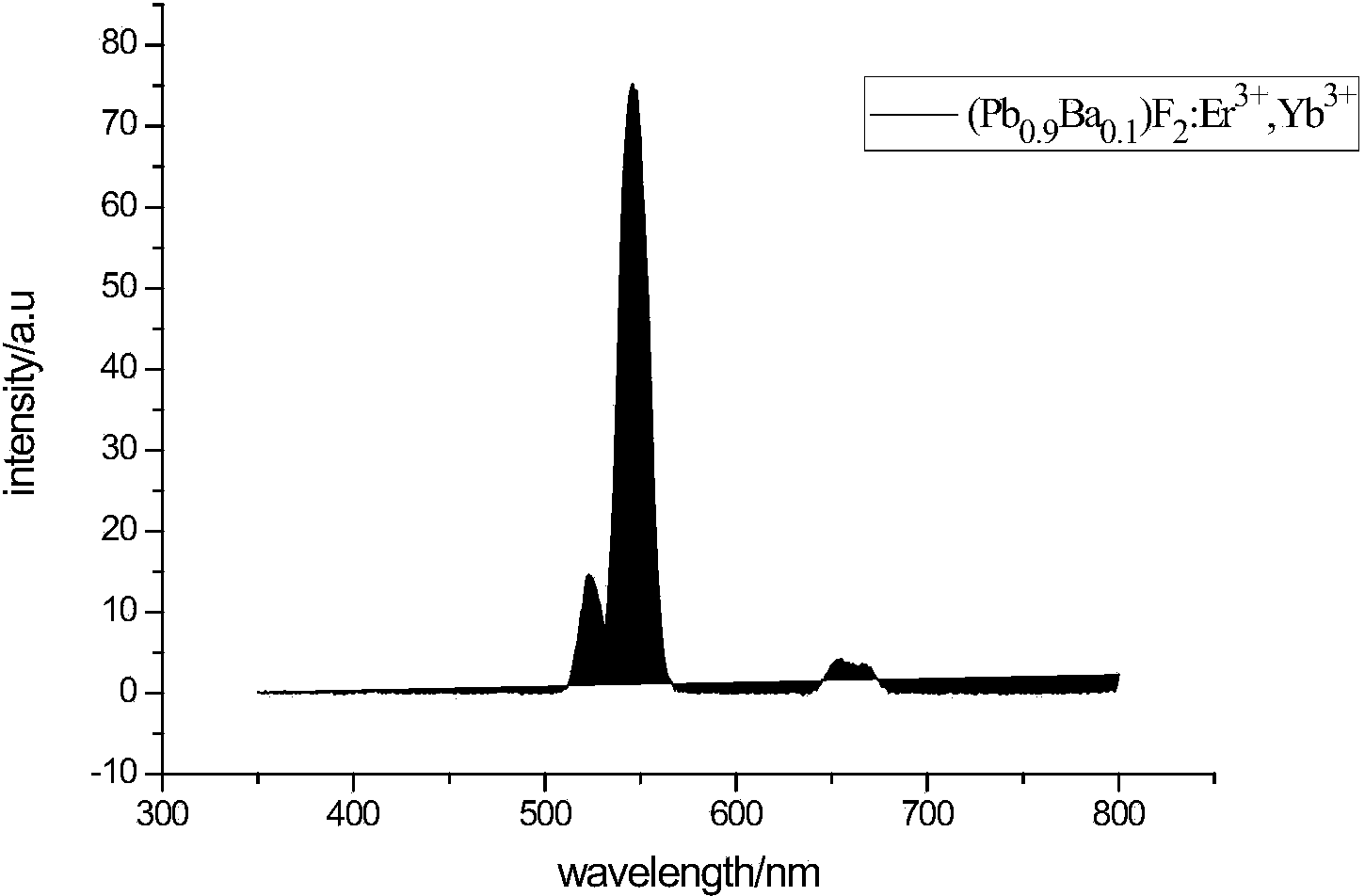
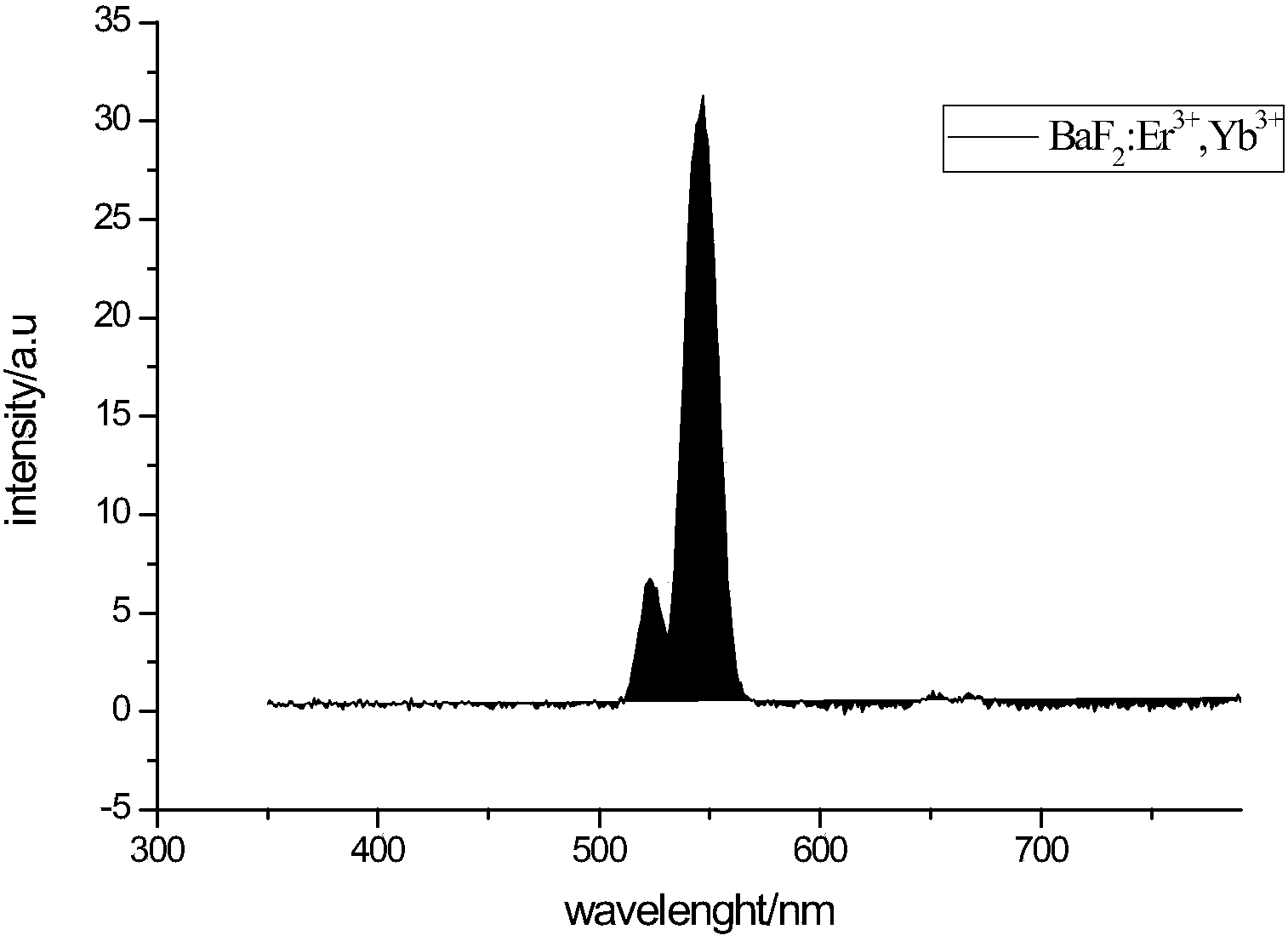
1064nm lead fluoride based up-conversion luminescence material and preparation method thereof
A technology of luminescent materials and lead fluoride, which is applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems of poor chemical stability and mechanical strength, and achieve the effect of loosening the requirements of the preparation equipment and process and reducing the preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Calculate according to the stoichiometric ratio and weigh 0.191g Er 2 o 3 and 1.773gYb 2 o 3 , and put it into a beaker; weigh 2.25ml of nitric acid with a concentration of 8mol / L, add Er 2 o 3 and Yb 2 o 3 In a beaker, the beaker is placed on a heating magnetic stirrer, and Er(NO 3 ) 3 and Yb(NO 3 ) 3 solution.
[0023] 2. Dissolve 8.928gPb(NO 3 ) 2 , to get Pb(NO 3 ) 2 solution.
[0024] 3. Weigh 4.115g of NaF, add deionized water, place it on a magnetic stirrer, stir and dissolve to obtain NaF solution, drop a drop of nitric acid to accelerate the dissolution.
[0025] 4. Mix the solutions obtained in step 1 and step 2, place the formed mixed solution on a magnetic stirrer and stir for 10 minutes, and adjust the pH value to 2.5; add the solution obtained in step 3 to the mixed solution and stir for 1 Hours, the fluorides of the reaction raw materials Yb, Er, and Pb are obtained through the reaction, and the various fluorides exist in the solution i...
Embodiment 2
[0032] 1. Calculate according to the stoichiometric ratio and weigh 0.191g Er 2 o 3 and 1.773gYb 2 o 3 , and put it into a beaker; weigh 2.25ml of nitric acid with a concentration of 8mol / L, add Er 2 o 3 and Yb 2 o 3 In a beaker, the beaker is placed on a heating magnetic stirrer, and Er(NO 3 ) 3 and Yb(NO 3 ) 3 solution.
[0033] 2. Dissolve 11.923gPb(NO 3 ) 2 and 1.045gBa(NO 3 ) 2 , to get Pb(NO 3 ) 2 solution and Ba(NO 3 ) 2 solution.
[0034] 3. Weigh 4.115g of NaF, add deionized water, place it on a magnetic stirrer, stir and dissolve to obtain NaF solution, drop a drop of nitric acid to accelerate the dissolution.
[0035] 4. Mix the solutions obtained in step 1 and step 2, place the formed mixed solution on a magnetic stirrer and stir for 10 minutes, and adjust the pH value to 2.5; add the solution obtained in step 3 to the mixed solution and stir for 1 Hours, the reaction raw materials Yb, Er, Pb fluoride and Ba fluoride are obtained through the rea...
Embodiment 3
[0042] 1. Weigh 3.941gYb 2 o 3 and 0.383gEr 2 o 3 , put them into beakers respectively; weigh 7.5ml and 0.75ml of nitric acid respectively, and add 2 o 3 and Er 2 o 3 Put it in a beaker with a heating magnetic stirrer to make it fully react, and finally obtain a transparent solution.
[0043] 2. Pour the two solutions into plastic cups respectively, and then add an excess of 40.0% analytically pure hydrofluoric acid to it to fully react.
[0044] 3. Put the substance obtained in step 2 into a centrifuge for separation, then pour off the liquid in the upper layer, add deionized water to stir, and then separate twice.
[0045] 4. Transfer the substance obtained in step 3 to a clean beaker, and dry it in an oven at 120°C for 10 hours to obtain ErF 3 and YbF 3 block samples.
[0046] 5. Weigh 7.013gBaF according to the stoichiometric ratio 2 , 1.035gYbF 3 , 0.112gErF 3 , 16%NaF, 8%Na 2 SiF 6 , put it into an agate mortar, grind it repeatedly to make it evenly mixed,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com