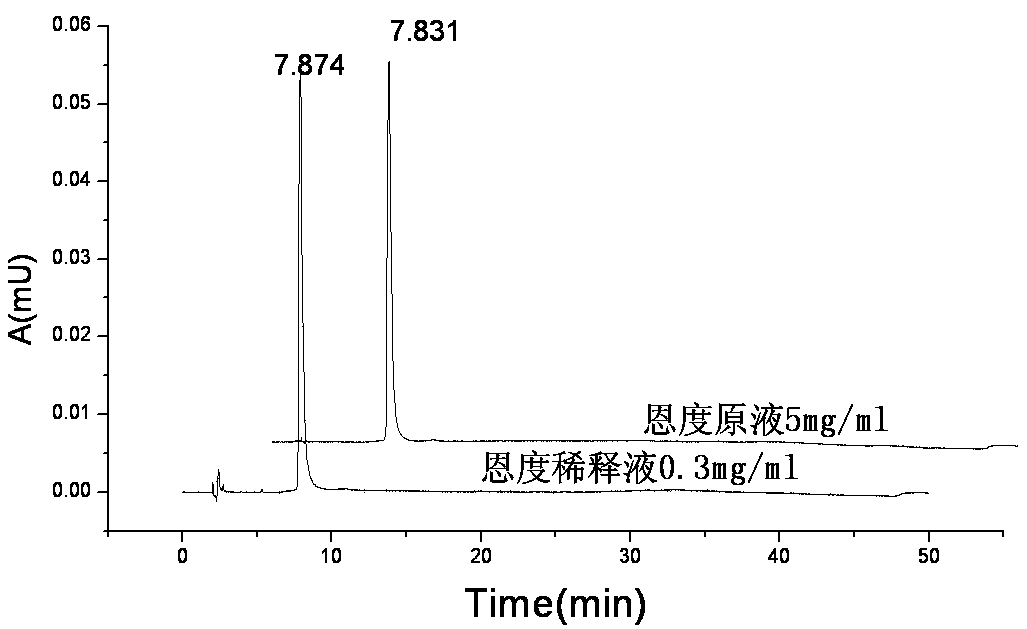
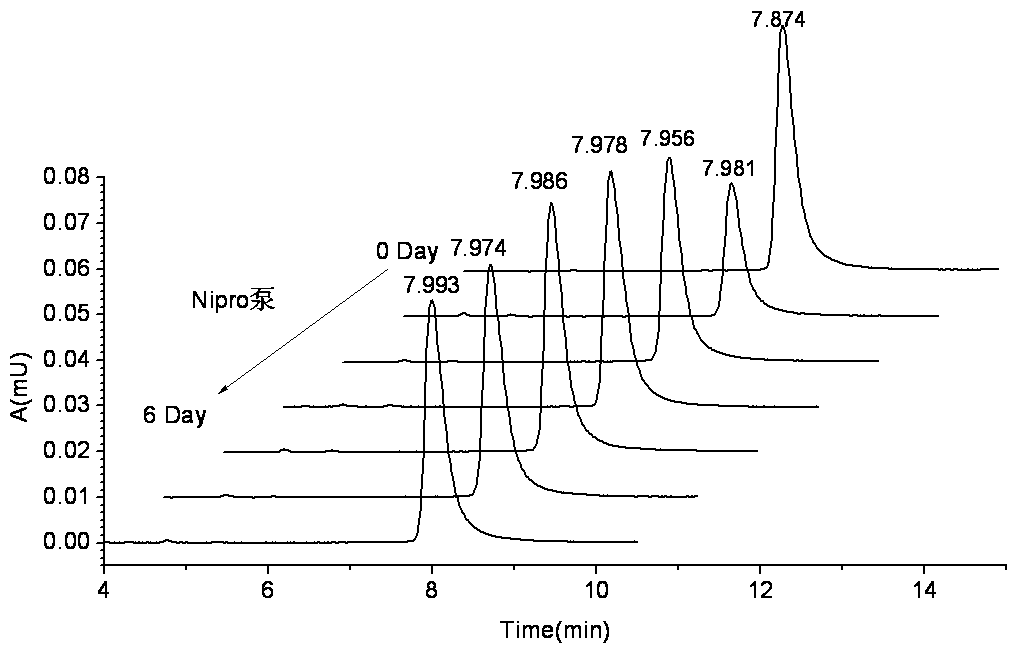
Recombinant human endostatin continuous infusion system
A technology of continuous drug administration and vascular endothelium, applied in the direction of devices introduced into the body, pressure infusion, etc., can solve the problems of excessive fluctuations in blood drug concentration and the inability to maintain a steady-state concentration, improve patient compliance, and solve long-term problems. Obstacles, effects that increase purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
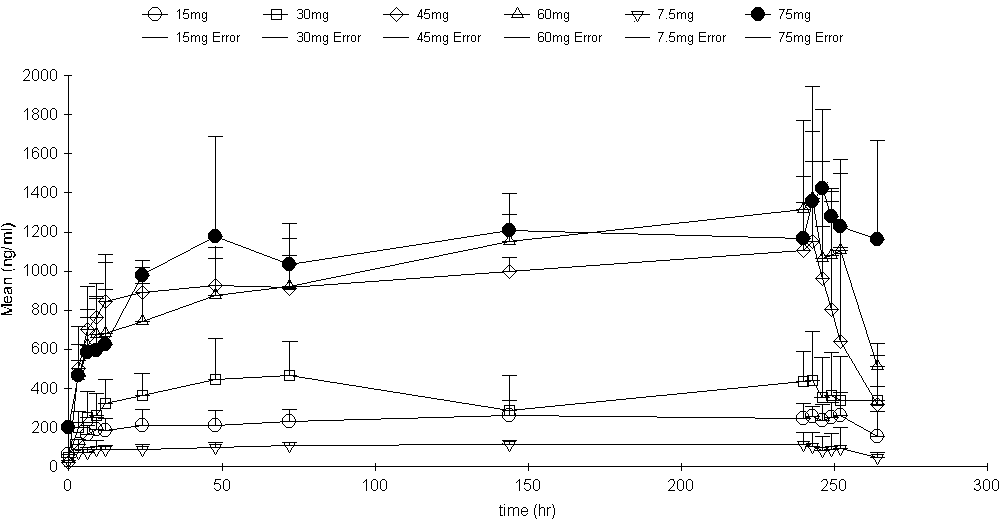
[0032] Pull out the needle plug of 2.5 Endostar syringes, fill the Endostar injection into the drug storage device of the Nipro pump, and then use the syringe to draw physiological saline to fill the drug storage device to replenish the drug solution to 240ml. Exhaust the air in the connecting tube, connect the intravenous indwelling catheter, fix it properly, set the parameters of the Nipro pump drive device to 2ml / hr, and pump Endostar into the patient at a loading dose of 0.3125mg / hr (7.5mg / day) for 120hr .
[0033]
Embodiment 2
[0035] Pull out the needle plugs of 5 Endostar syringes, fill the Endostar injection into the drug storage device of the Nipro pump, and then use the syringe to draw physiological saline to fill the drug storage device to replenish the drug solution to 240ml. Exhaust the air in the connecting tube, connect the intravenous indwelling catheter, fix it properly, set the parameters of the Nipro pump drive device to 2ml / hr, and pump Endostar into the patient at a loading dose of 0.625mg / hr (15mg / day) for 120hr.
[0036]
Embodiment 3
[0038] Pull out the needle plugs of 10 Endostar syringes, fill the Endostar injection into the drug storage device of the Baxter pump, and then use the syringe to draw physiological saline and fill the drug storage device to replenish the drug solution to 240ml. Exhaust the air in the connecting tube, connect the intravenous indwelling catheter, fix it properly, set the parameters of the Baxter pump drive device to 2ml / hr, and pump Endostar into the patient at a loading dose of 1.25mg / hr (30mg / day) for 120hr.
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com