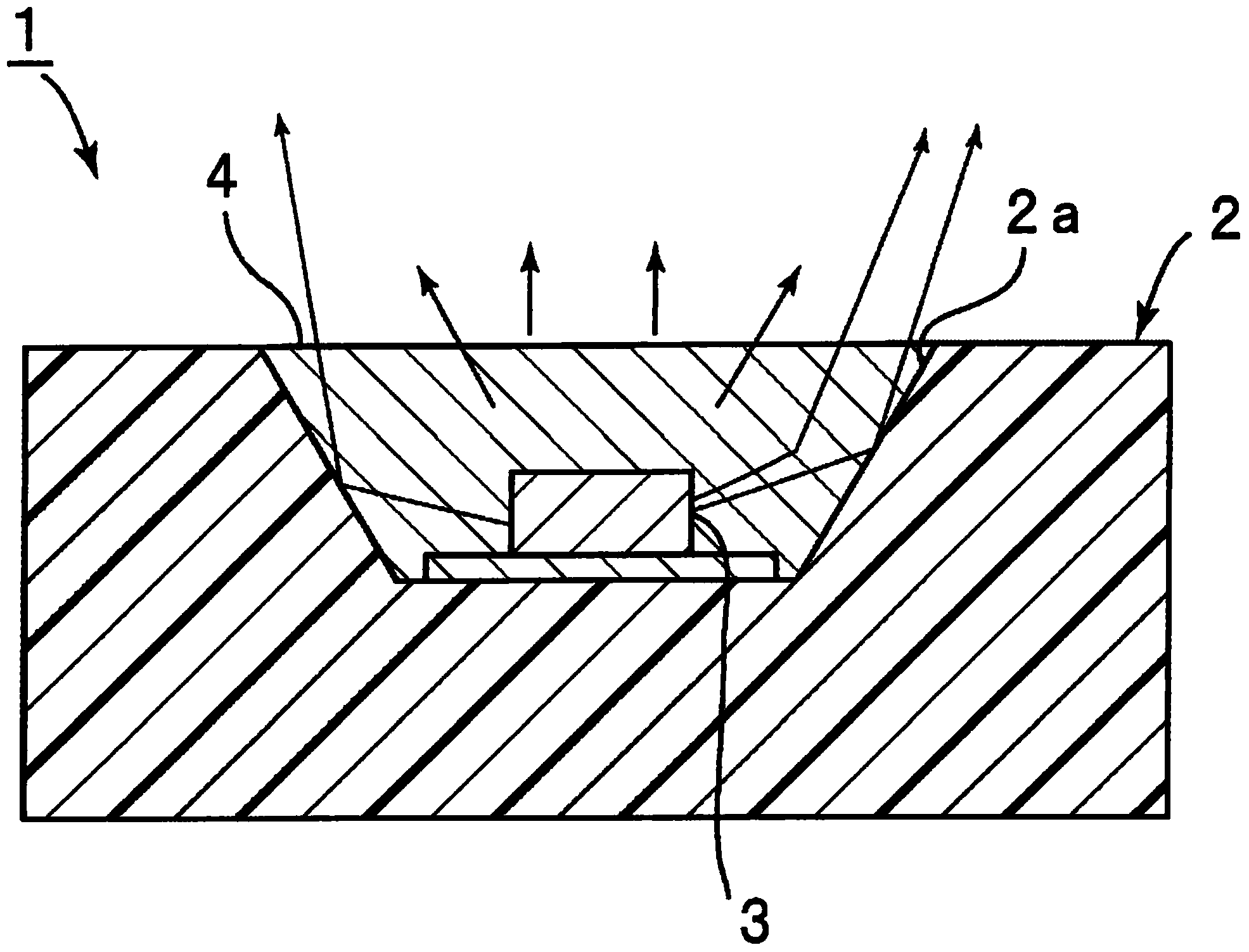
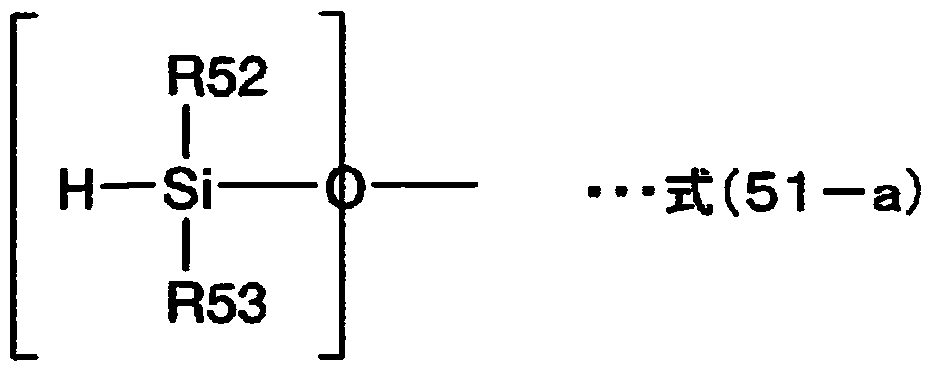
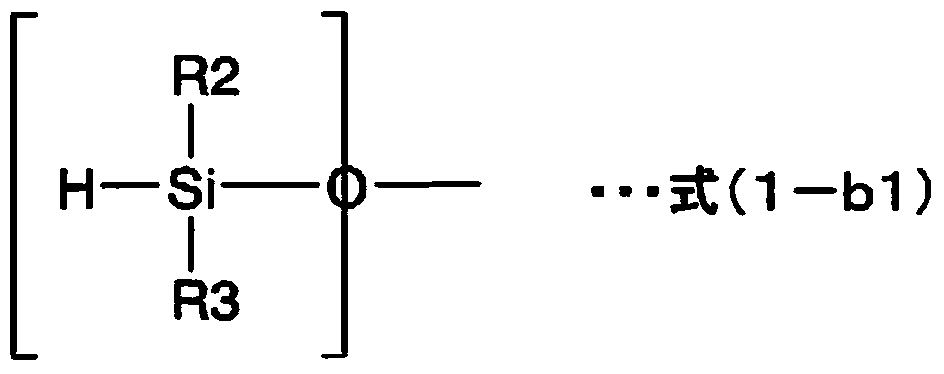
Sealing agent for optical semiconductor devices, and optical semiconductor device
A technology for optical semiconductor devices and sealants, which is applied in the direction of semiconductor devices, semiconductor/solid-state device parts, electrical components, etc., can solve problems such as easy dust adhesion, component adhesion, and reduced productivity of optical semiconductor devices, so as to improve durability Thermal properties, cooling and heating cycle characteristics, stickiness suppression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0261] (Synthesis example 1) Synthesis of the first organopolysiloxane represented by formula (1A)
[0262] Add 476 g of dimethyldimethoxysilane and 1,3-divinyl-1,1,3,3-tetramethyldisilazane to a 1000 mL detachable flask equipped with a thermometer, a dropping device, and a stirrer 5.3 g of oxane was stirred at 50°C. A solution obtained by dissolving 2.2 g of potassium hydroxide in 144 g of water was slowly added dropwise thereto, and after the dropwise addition, it was stirred and reacted at 50° C. for 6 hours to obtain a reaction liquid. Next, volatile components were removed under reduced pressure, and 2.4 g of acetic acid was added to the reaction liquid, followed by heating under reduced pressure. Then, potassium acetate was removed by filtration to obtain a polymer (A).
[0263] The number average molecular weight of the obtained polymer (A) was 5830. use 29 As a result of identification of the chemical structure by Si-NMR, the polymer (A) has the following average c...
Synthetic example 2
[0267] (Synthesis example 2) Synthesis of the first organopolysiloxane represented by formula (1A)
[0268] Add 486 g of dimethyldimethoxysilane and 1,3-divinyl-1,1,3,3-tetramethyldisilazane to a 1000 mL detachable flask equipped with a thermometer, a dropping device, and a stirrer 2.7 g of oxane was stirred at 50°C. A solution obtained by dissolving 2.2 g of potassium hydroxide in 144 g of water was slowly added dropwise thereto, and after the dropwise addition, it was stirred and reacted at 50° C. for 6 hours to obtain a reaction liquid. Next, volatile components were removed under reduced pressure, and 2.4 g of acetic acid was added to the reaction liquid, followed by heating under reduced pressure. Then, potassium acetate was removed by filtration to obtain a polymer (B).
[0269] The number average molecular weight of the obtained polymer (B) was 37,400. use 29 As a result of Si-NMR identification of the chemical structure, the polymer (B) has the following average co...
Synthetic example 3
[0272] (Synthesis Example 3) Synthesis of the First Organopolysiloxane Represented by Formula (1A)
[0273] Add 488 g of dimethyldimethoxysilane and 1,3-divinyl-1,1,3,3-tetramethyldisilane to a 1000 mL detachable flask equipped with a thermometer, a dropping device, and a stirrer 1.2 g of oxane was stirred at 50°C. A solution obtained by dissolving 2.2 g of potassium hydroxide in 144 g of water was slowly added dropwise thereto, and after the dropwise addition, it was stirred and reacted at 50° C. for 6 hours to obtain a reaction liquid. Next, volatile components were removed under reduced pressure, and 2.4 g of acetic acid was added to the reaction liquid, followed by heating under reduced pressure. Then, potassium acetate was removed by filtration to obtain a polymer (C).
[0274] The number average molecular weight of the obtained polymer (C) was 82800. use 29 As a result of identification of the chemical structure by Si-NMR, the polymer (C) has the following average co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com