Quinazoline derivatives and their preparation and use
A quinazoline and derivative technology, applied in the field of drug synthesis, can solve the problems of increasing the difficulty of curing and the probability of recurrence, and achieve good inhibitory activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
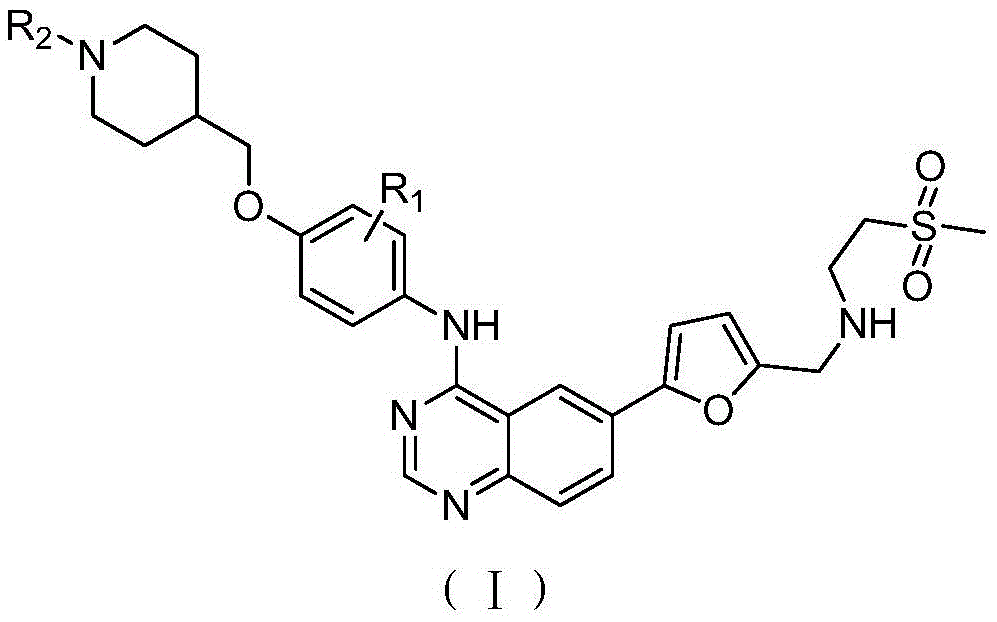
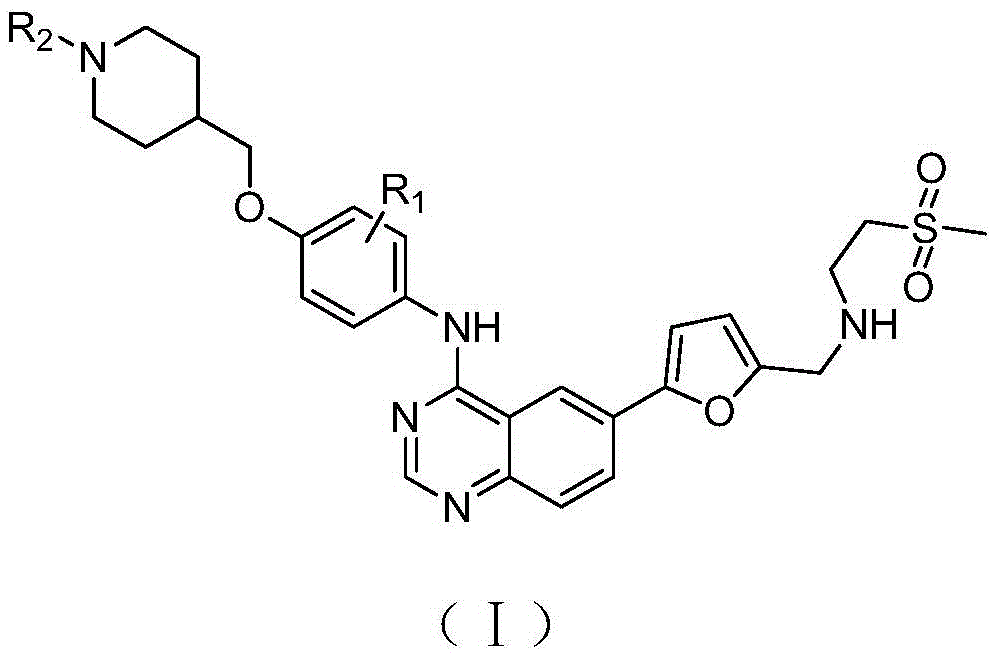
[0038] 1-(4-((4-((6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)furan-2-yl)quinazolin-4-yl)amino) Synthesis of phenoxy)methyl)piperidin-1-yl)ethanone (Ⅰ-1)
[0039]
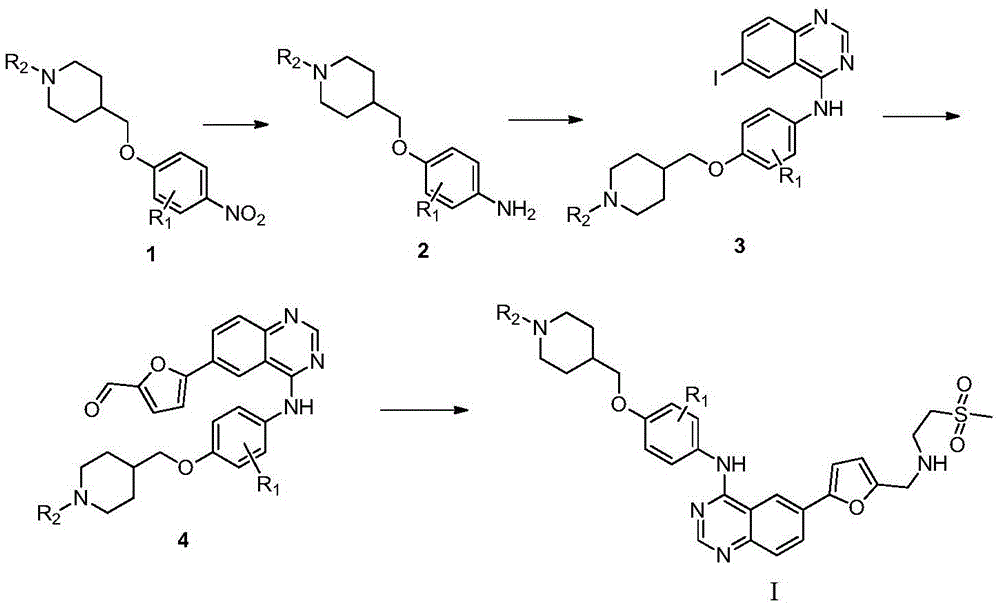
[0040] Add 500 mL of ethanol and 5 mL of sulfuric acid into a 1000 mL four-neck reaction flask, then add 50 g of 1-(4-((4-nitrophenoxy)methyl)piperidin-1-yl)ethanone 1, and heat up to reflux. Add 60g of reduced iron powder several times in small amounts, and continue the reflux reaction for 3 hours after adding the iron powder. Cool to room temperature and add ammonia water dropwise to adjust the pH to about 9, filter, remove iron sludge, and wash with water. The filtrate was adjusted to pH=4 with hydrochloric acid, the product was precipitated, filtered, and the filter cake was dried to obtain 36.8 g of 1-(4-((4-aminophenoxy)methyl)piperidin-1-yl)ethanone 2. 1 HNMR(400MHz,DMSO):δ1.34-1.59(m,4H,CH 2 ),2.00(m,1H,CH),2.36(s,3H,CH 3),3.20-3.40(m,4H,CH 2 ),3.90(d,2H,CH 2 ),6.50-6.80(dd,4H,ArH); ESI-MS: m / z249...
Embodiment 2
[0045] Cyclopropyl 1-(4-((4-((6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)furan-2-yl)quinazolin-4-yl )amino)phenoxy)methyl)piperidin-1-yl)methanone (Ⅰ-2) synthesis
[0046]
[0047] Prepared according to the same method as in Example 1, cyclopropyl (4-((4-nitrophenoxy) methyl) piperidin-1-yl) ketone is reduced by iron powder to obtain compound (4-(( 4-aminophenoxy)methyl)piperidin-1-yl)cyclopropylmethanone, reacted with 4-chloro-6-iodoquinazoline, and then reacted with 5-formyl-2-furylboronic acid, Finally react with 2-methanesulfonylethylamine to obtain the target compound I-2, 1 HNMR(400MHz,DMSO):δ0.72-0.93(m,4H,CH 2 ),1.15-1.17(m,1H,CH),1.34-1.59(m,4H,CH 2 ),2.00(m,1H,CH),2.83(s,3H,CH 3 ),3.11(m,2H,CH 2 ),3.20-3.40(m,4H,CH 2 ),3.53(m,2H,CH 2 ),3.67(s,2H,CH 2 ),3.90(d,2H,CH 2 ),6.39(d,1H,ArH),6.74(d,2H,ArH),7.35(d,1H,ArH),7.60(d,2H,ArH),7.93(d,1H,ArH),8.20(m ,2H,ArH),8.60(s,1H,ArH);ESI-MS:m / z604[M+H] + .
Embodiment 3
[0049] 6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)furan-2-yl)-N-(4-((1-(methylsulfonyl)piperidin-4-yl Synthesis of )methoxy)phenyl)quinazolin-4-amine (Ⅰ-3)
[0050]
[0051] Prepared according to the same method as in Example 1, 1-methylsulfonyl-(4-((4-nitrophenoxy)methyl)piperidine was reduced by iron powder to obtain compound (4-((1-methylsulfonyl) Acyl)piperidin-4-yl)methoxyaniline, then reacted with 4-chloro-6-iodoquinazoline, then with 5-formyl-2-furylboronic acid, and finally with 2-methanesulfonylethylamine The reaction gives the target compound I-3, 1 HNMR(400MHz,DMSO):δ1.34-1.59(m,4H,CH 2 ),2.00(m,1H,CH),2.83(s,3H,CH 3 ),2.96(s,3H,CH 3 ),3.11(m,2H,CH 2 ),3.20-3.40(m,4H,CH 2 ),3.53(m,2H,CH 2 ),3.67(s,2H,CH 2 ),3.90(d,2H,CH 2 ),6.39(d,1H,ArH),6.74(d,2H,ArH),7.35(d,1H,ArH),7.60(d,2H,ArH),7.93(d,1H,ArH),8.20(m ,2H,ArH),8.60(s,1H,ArH);ESI-MS:m / z614[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com