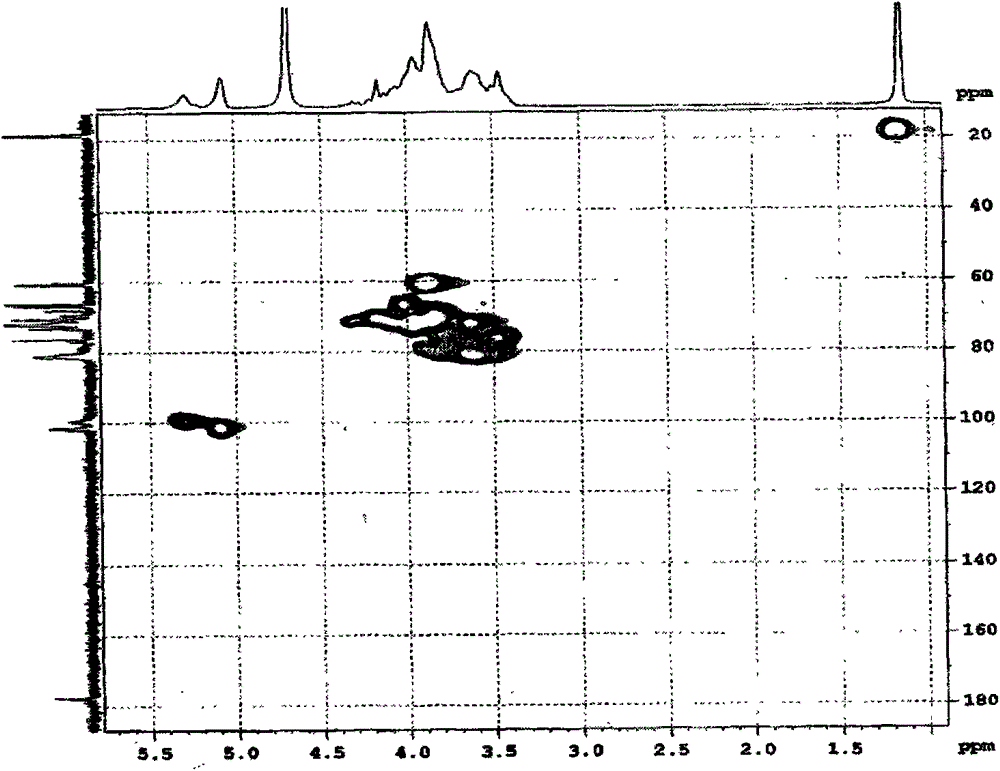
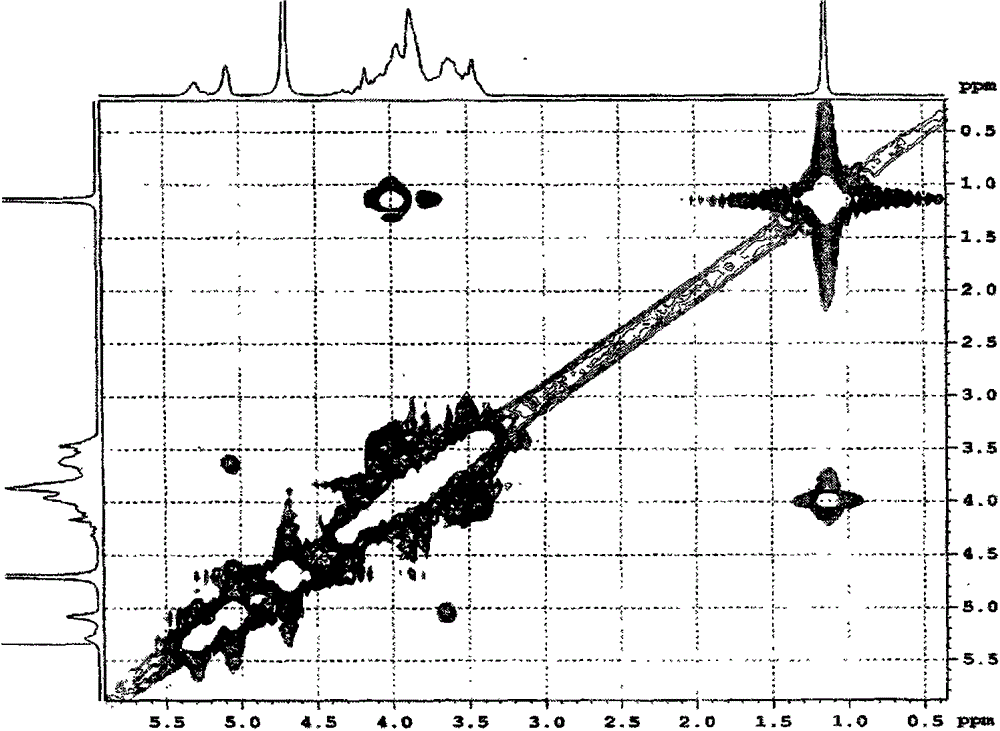
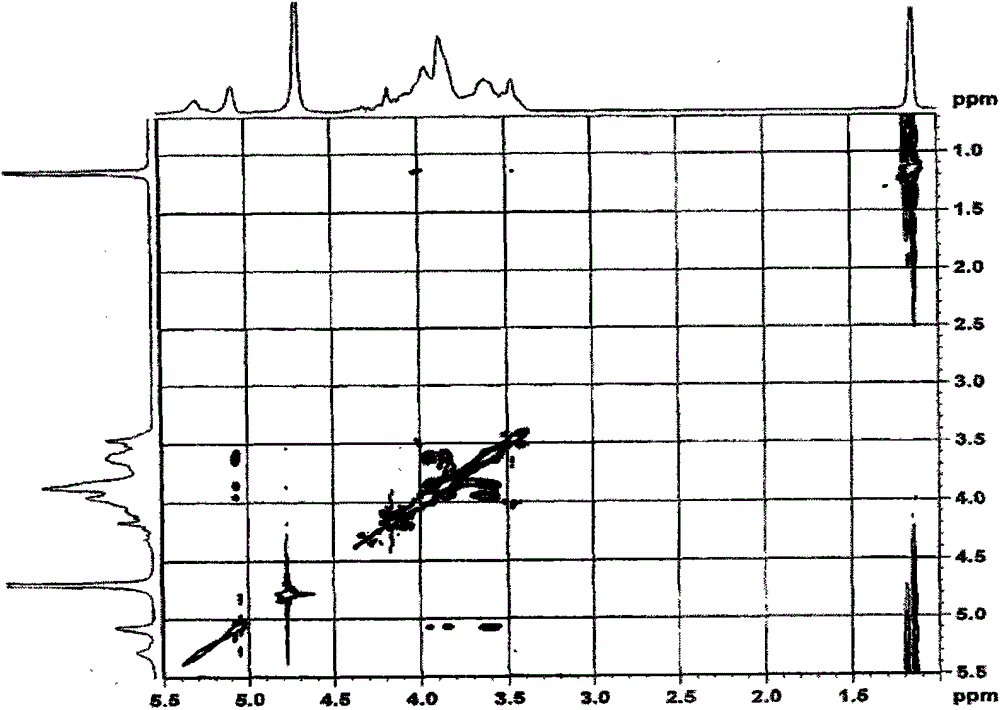
Carboxymethyl-hydroxypropyl-β-cyclodextrin and its preparation method
A carboxymethyl and cyclodextrin technology, which is applied in the field of carboxymethyl-hydroxypropyl-β-cyclodextrin and its preparation, can solve the problems of high dosage of prescription auxiliary materials, limited practicability, influence on cyclodextrin, and the like, Achieve good solubility, appropriate drug inclusion ability, and small hemolysis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The three-necked round-bottomed flask is equipped with a constant pressure funnel, a reflux condenser, and a thermometer. Add 50ml of pure water into it, start stirring, and then slowly add 0.02mol β-cyclodextrin (22.7g) and 0.3mol solid NaOH (12.0g) under successive stirring. ), the system was dissolved and then heated slowly to keep at 80°C, then slowly added 0.08mol ethyl chloroacetate (9.8g) dropwise for about 4 hours, continued to stir and reflux for 2 hours and then cooled to room temperature. Control the temperature of the water bath to <20°C, slowly add 0.14mol of 1,2-propylene oxide (8.1g) dropwise to the system under stirring, and complete the addition in about 3 hours, continue to stir and react for 5 hours under the condition of the water bath temperature pH to neutral, filter, pour the filtrate into a dialysis bag, and dialyze to remove residual β-cyclodextrin and 1,2-propanediol and glycolic acid (sodium) produced by the reaction. After changing water and ...
Embodiment 2
[0079] Add 50ml of pure water to a three-necked round-bottom flask equipped with a constant pressure funnel, reflux condenser, and thermometer, start stirring, and then slowly add 0.02mol β-cyclodextrin (22.7g) and 0.14mol solid NaOH (12.0g) in sequence After the system is dissolved, control the temperature of the water bath to 1 HNMR verifies that the average degree of substitution of carboxymethyl is 2.1, and the average degree of substitution of hydroxypropyl is 5.8. The product is abbreviated as CM 2 -HP 6 -β-CD.
Embodiment 3
[0081] It is basically the same as Example 1, but the feeding amounts of NaOH, ethyl chloroacetate, and 1,2-propylene oxide are respectively 0.22, 0.04, and 0.05 mol. Finally, the product yield is 98.5%. 1 HNMR verifies that the average substitution degree of carboxymethyl group is 1.2, and the average substitution degree of hydroxypropyl group is 1.8. The product is abbreviated as CM 1 -HP 2 -β-CD.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com