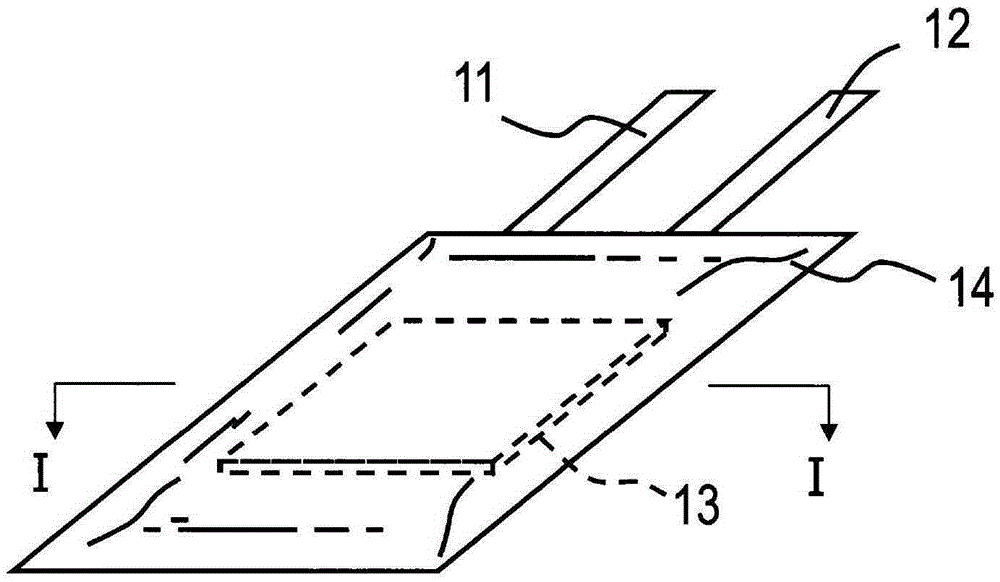
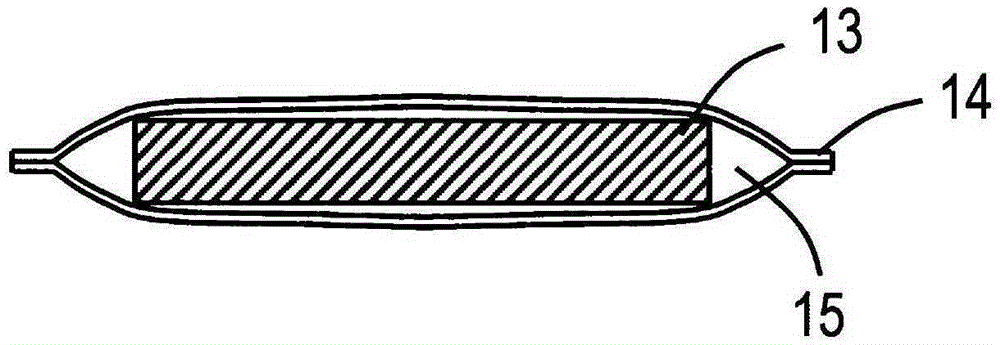
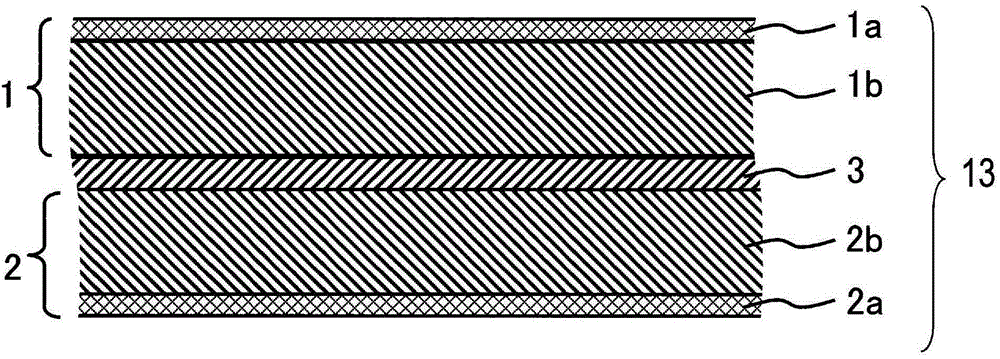
Non-aqueous electrolyte secondary battery and manufacturing method thereof
一种非水电解质、二次电池的技术,应用在非水电解质蓄电池、电解质蓄电池制造、二次电池等方向,能够解决正极电位变化大、正极单极循环劣化速度变高等问题,达到循环特性提高、抑制循环劣化、循环特性优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0085]
[0086] First, as the positive electrode active material, LiNi 0.80 co 0.15 Al 0.05 o 2 . LiNi 0.80 co 0.15 Al 0.05 o 2 The modulation method is as follows.
[0087] A predetermined ratio of cobalt sulfate was added to an aqueous nickel sulfate solution having a concentration of 1 mol / liter to prepare an aqueous metal salt solution. While stirring the aqueous metal salt solution at a low speed while maintaining the temperature at 50° C., an alkali solution containing 30% by weight of sodium hydroxide was added dropwise to adjust the pH to 12 to obtain hydroxide precipitates. After filtering and washing this precipitate with water, it dried by heating at 80 degreeC in air.
[0088] Next, the obtained hydroxide was stirred in water in a reaction tank at 30°C, and a predetermined amount of NaAlO was added to the reaction tank. 2And stir well. Thereafter, the solution in the reaction tank was neutralized to pH 9 using sulfuric acid. As a result, aluminum hydr...
Embodiment 2)
[0108] A battery was produced in the same manner as in Example 1 except that the content of graphite fluoride in the negative electrode mixture was different. The negative electrode mixture is based on the weight ratio of Li 4 Ti 5 o 12 :VGCF:PVdF:(CF) n =100:5:4:0.82, add an appropriate amount of N-methyl-2-pyrrolidone (NMP) to stir and mix to form a slurry-like negative electrode mixture.
Embodiment 3)
[0112] A battery was produced in the same manner as in Example 1 except that the negative electrode mixture was different. With the Li that the method identical with embodiment 1 is made 4 Ti 5 o 12 , Acetylene black (AB) as a conductive agent, polyvinylidene fluoride (PVdF) as a binder, and graphite fluoride (manufactured by Daikin Industries, (CF) n ) to Li by weight ratio 4 Ti 5 o 12 :AB:PVdF:(CF) n =100:5:4:2.9, add an appropriate amount of N-methyl-2-pyrrolidone (NMP) to stir and mix to form a slurry-like negative electrode mixture.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| surface pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com