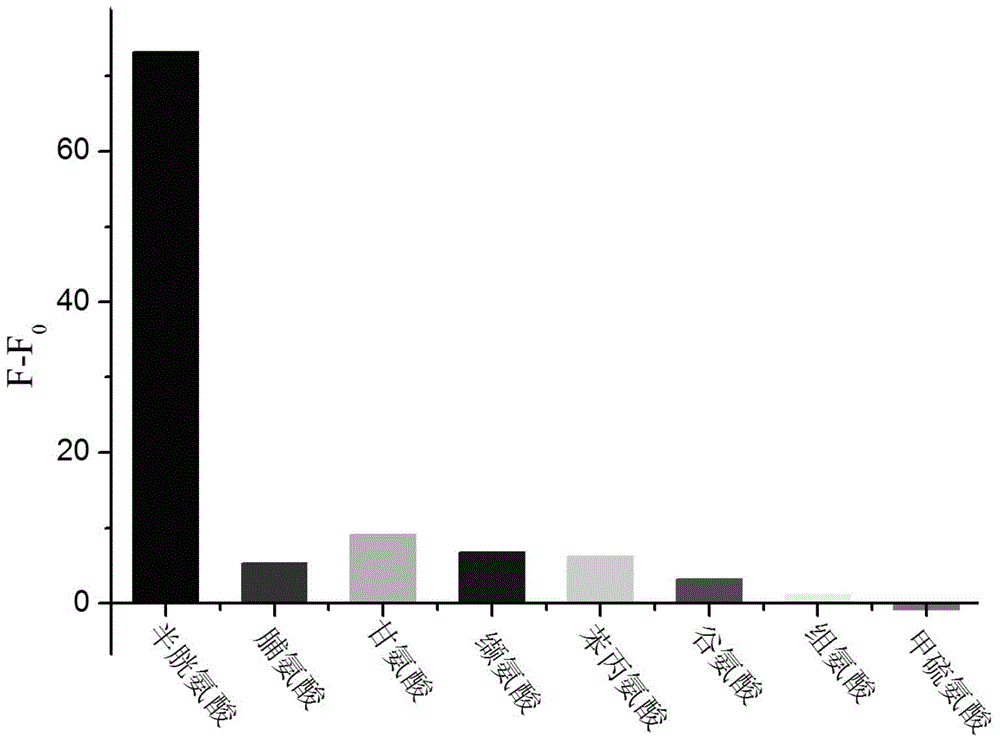
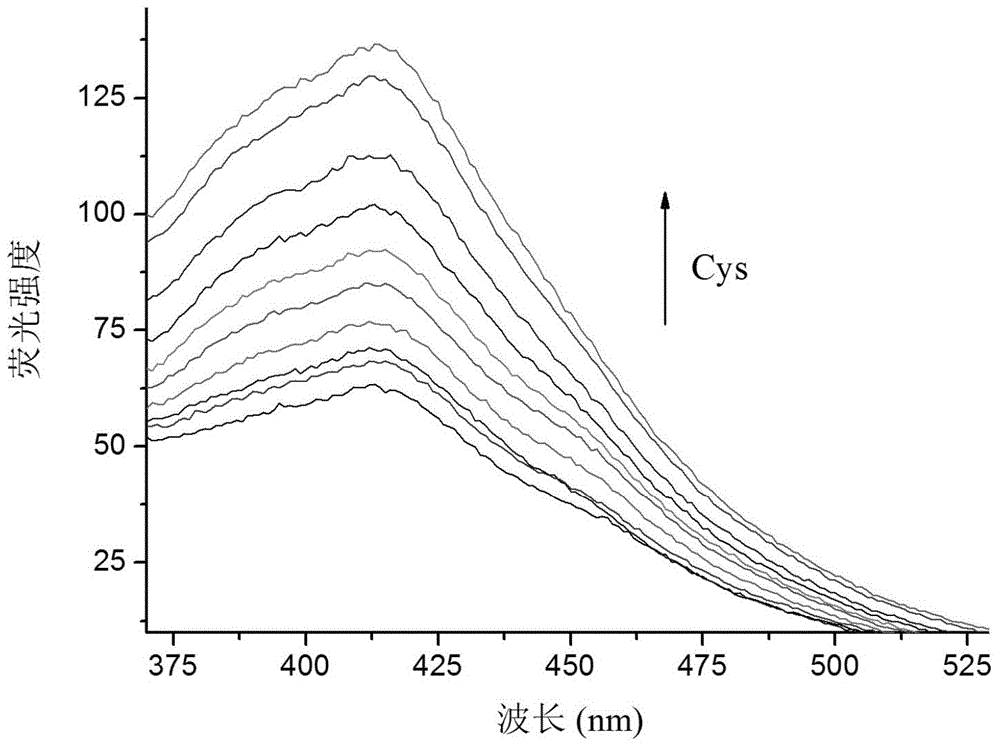
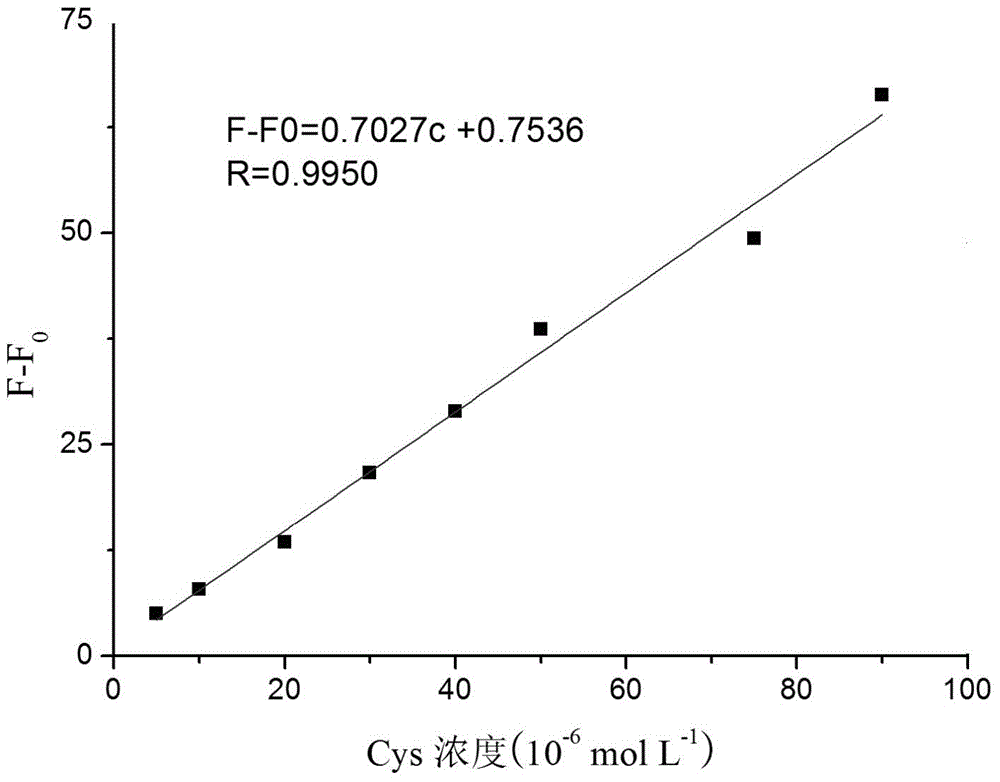
A kind of coumarin derivative and its preparation method and application
A technology of coumarin derivatives and hydroxycoumarin, which is applied in biological testing, chemical instruments and methods, material inspection products, etc., can solve the problems of enhanced fluorescence intensity and no significant fluorescence response, and achieve the effect of enhanced fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 coumarin derivatives
[0025] (1) In a 100mL reaction flask, add 50mL of concentrated sulfuric acid, cool to 0°C with an ice-water bath, start stirring, add 5.5g (50mmol) resorcinol, and then dropwise add 6.8g (41.3mmol) 4-chloroacetyl Ethyl acetate, the rate of addition was controlled to keep the temperature of the reaction system at 0±2°C, and the reaction was stirred overnight at room temperature after the addition was complete. The reaction solution was poured into ice water, and a white solid was precipitated, filtered, washed with water, and dried to obtain 6.9 g of 4-chloromethyl-7-hydroxycoumarin, with a yield of 79.4%.
[0026] (2) In a 2L reaction flask, add 6.9g (32.8mmol) of 4-chloromethyl-7-hydroxycoumarin and 900mL of water, heat to reflux for 36 hours, cool, and precipitate a white solid, filter, wash with water, After drying, 5.9 g of 4-hydroxymethyl-7-hydroxycoumarin was obtained, with a yield of 93.7%.
[0027] (3) In ...
Embodiment 2
[0033] The preparation of embodiment 2 coumarin derivatives
[0034] (1) Same as embodiment 1;
[0035] (2) Same as embodiment 1;
[0036] (3) Same as embodiment 1;
[0037] (4) In a 10mL reaction flask, add 2mL water and 0.38g (2mmol) 7-hydroxycoumarin-4-carbaldehyde, start stirring, then add 0.198g (3mmol) malononitrile and 32μL 1-methylimidazole, The reaction was stirred at room temperature for 12 hours. Filter, wash with water, and dry to obtain 0.368g of 2,2-dicyano-3-(7-hydroxy-4-coumarinyl)oxirane, with a yield of 72.6%.
Embodiment 3
[0038] The preparation of embodiment 3 coumarin derivatives
[0039] (1) Same as embodiment 1;
[0040] (2) Same as embodiment 1;
[0041] (3) Same as embodiment 1;
[0042] (4) In a 10mL reaction flask, add 2mL water and 0.38g (2mmol) 7-hydroxycoumarin-4-carbaldehyde, start stirring, then add 0.264g (4mmol) malononitrile and 48μL 1-methylimidazole, The reaction was stirred at room temperature for 12 hours. Filter, wash with water, and dry to obtain 0.356g of 2,2-dicyano-3-(7-hydroxy-4-coumarinyl)oxirane, with a yield of 70.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com