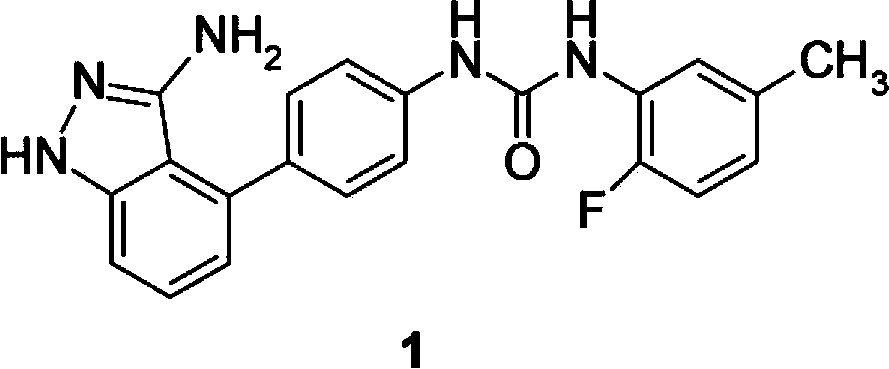
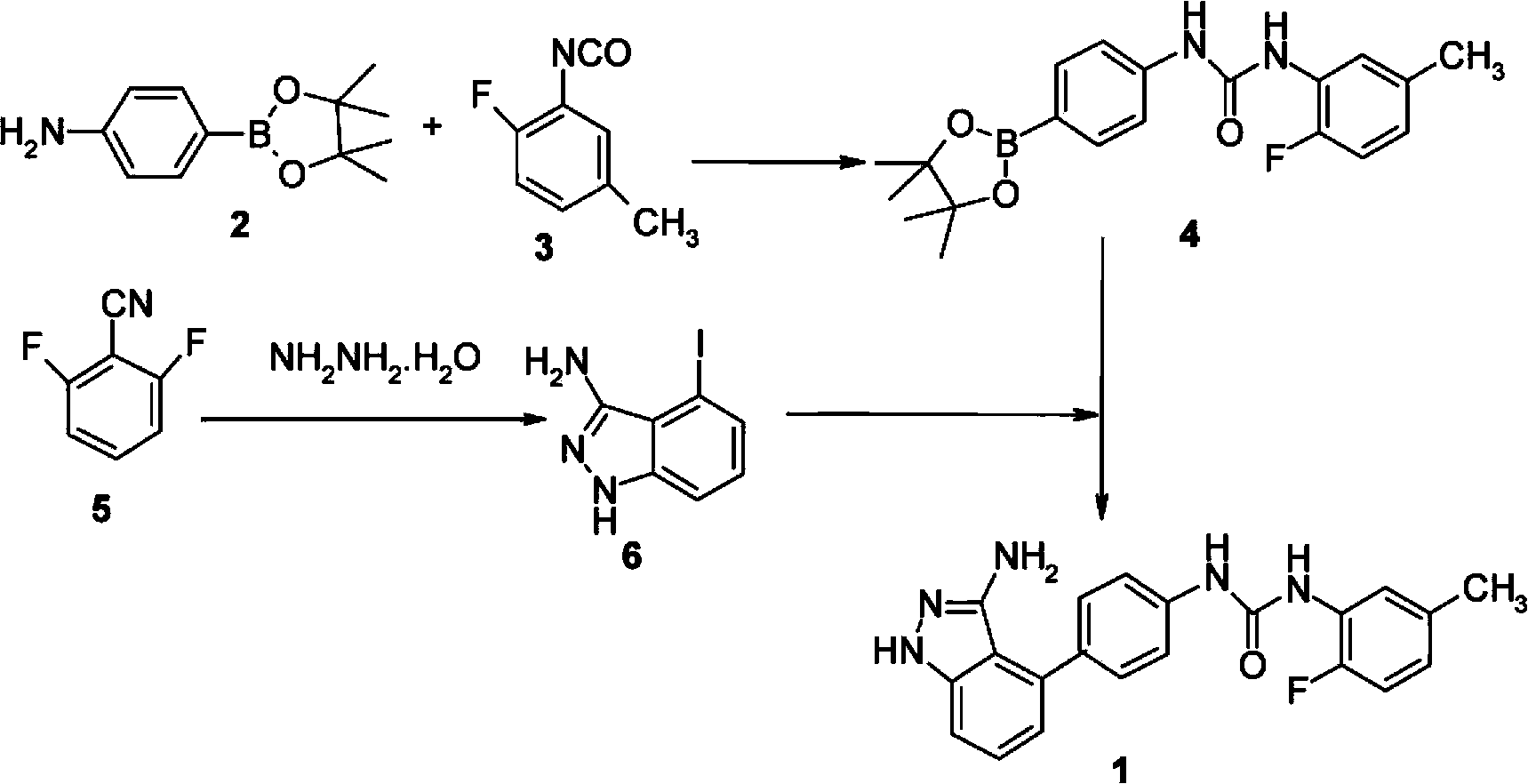
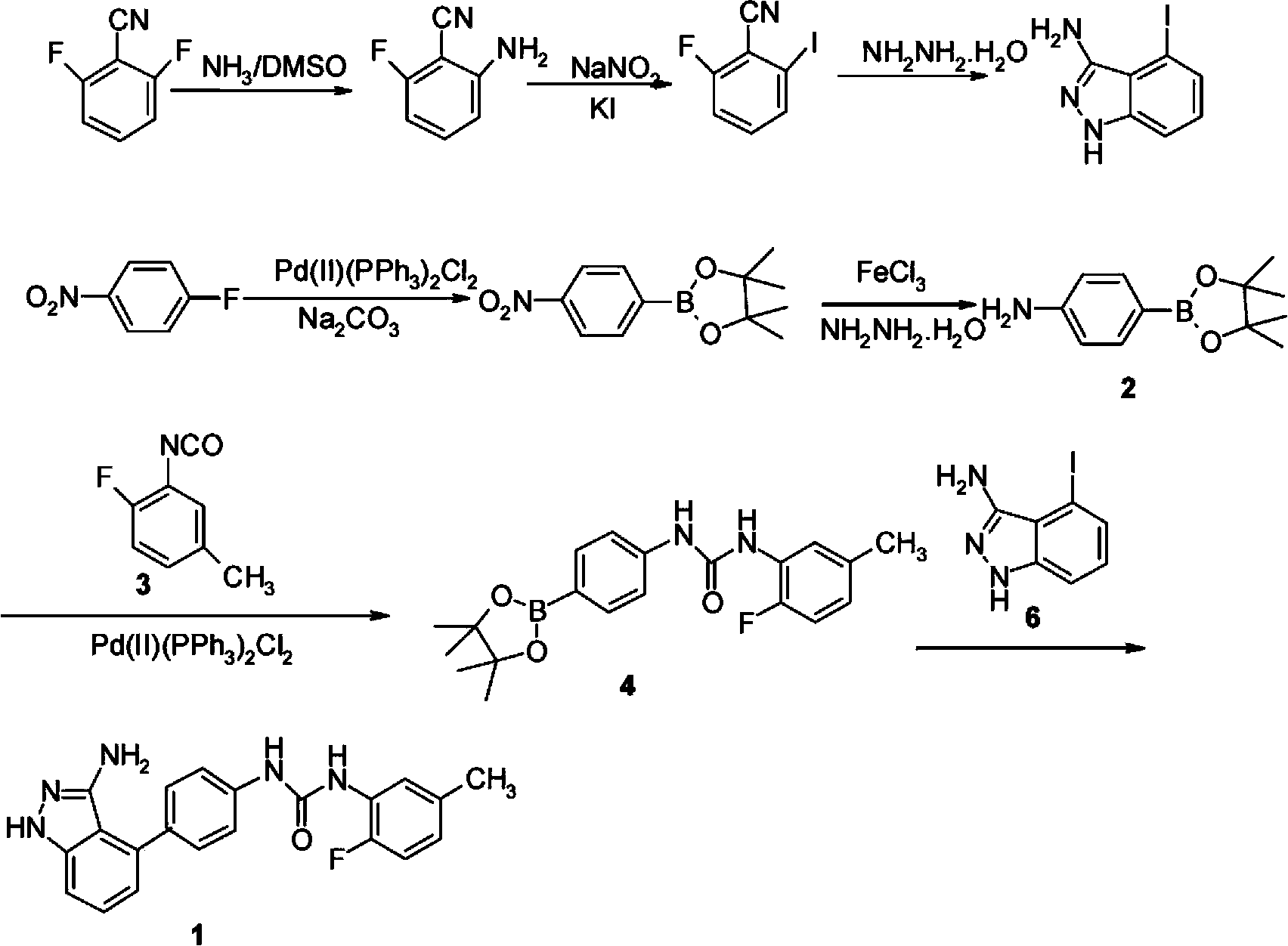
Preparation method of N-(4-(3-amino-1H-indazol-4-yl) phenyl)-N'-(2-fluoro-5-methylphenyl) urea and intermediate thereof
An amino and alkyl technology, applied in the field of drug synthesis, can solve the problems of long route, expensive raw materials, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] In the preparation method of the present invention, each reaction is usually carried out in an inert solvent at a reaction temperature of -20°C to reflux temperature (preferably -10°C to 100°C). The reaction time is usually 0.1-24 hours, preferably 0.5-18 hours.
[0102] The preparation of formula I compound
[0103] Compounds of formula I can be prepared as follows:
[0104]
[0105] In the presence of a base, the compound of formula III reacts with the compound of formula IV to form the compound of formula V;
[0106] The compound of formula V is then reacted with a boron reagent in the presence of a base and a palladium catalyst to form a compound of formula I.
[0107] The starting compounds of formula III and formula IV can be prepared by conventional methods or can be purchased.
[0108] In the present invention, representative boron reagents include (but are not limited to): diboron dioxide, tetramethoxydiboronate, tetraethoxydiboronate, tetra-n-butoxydibor...
Embodiment 1
[0120] Example 1 Preparation of compound 1-I
[0121]
[0122] Step A:
[0123] Dissolve triphosgene (8.9g, 30.0mmol) in 100mL ethyl acetate, add triethylamine (10.1g, 99.8mmol), stir and cool down to -10~-5°C, slowly add 2,5-dichloro A solution of aniline (8.1 g, 50.0 mmol) in ethyl acetate (80 mL) was added dropwise for about 0.5-1 hour. After the dropwise addition was completed, the temperature was raised to 50° C. and the reaction was stirred for 1-2 hours. TLC detected that the reaction of the raw materials was complete, cooled to room temperature, and the reaction solution was directly put into the next reaction.
[0124] Step B:
[0125] The temperature of the reaction solution in the previous step was raised to 25-30°C, and a solution of p-bromoaniline (6.9g, 40.1mmol) and diisopropylethylamine (6.5g, 50.3mmol) in ethyl acetate (50mL) was added dropwise to the reaction solution , dropwise for about 0.5-1 hour. After the dropwise addition, continue to control th...
Embodiment 2
[0128] The preparation of embodiment 2 compound 2-I
[0129]
[0130] Step A:
[0131] Dissolve triphosgene (14.8g, 100.0mmol) in 100mL of tetrahydrofuran, add diisopropylethylamine (13.4g, 103.7mmol), stir and cool down to -10~-5°C, slowly add 1-amino-2 , a solution of 4-dimethylbenzene (12.1 g, 100.0 mmol) in tetrahydrofuran (120 mL) was added dropwise for about 0.5-1 hour. After the dropwise addition, the temperature was raised to reflux and the reaction was stirred for 1-2 hours. TLC detected that the reaction of the raw materials was complete, cooled to room temperature, and the reaction solution was directly put into the next reaction.
[0132] Step B:
[0133] The reaction solution in the previous step was heated to 25-30°C, potassium carbonate (13.8g, 100.0mmol) was added, and a solution of p-bromoaniline (17.2g, 100.0mmol) in tetrahydrofuran (50mL) was added dropwise to the reaction solution, and about 0.5-1 hour. After the dropwise addition, continue to contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com