Salicylamide derivative crystal
A technology of compounds and diffraction peaks, applied in the field of crystallization of salicylic acid amide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
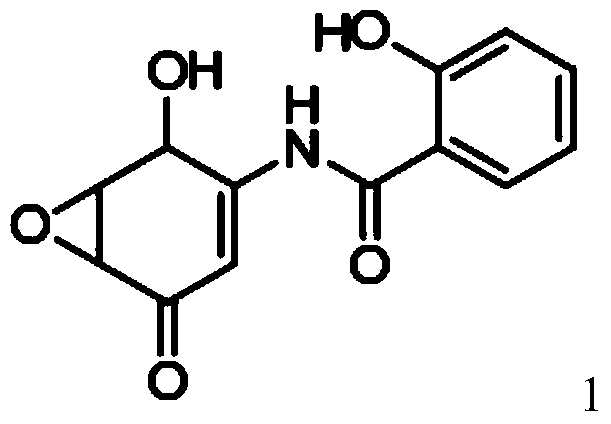
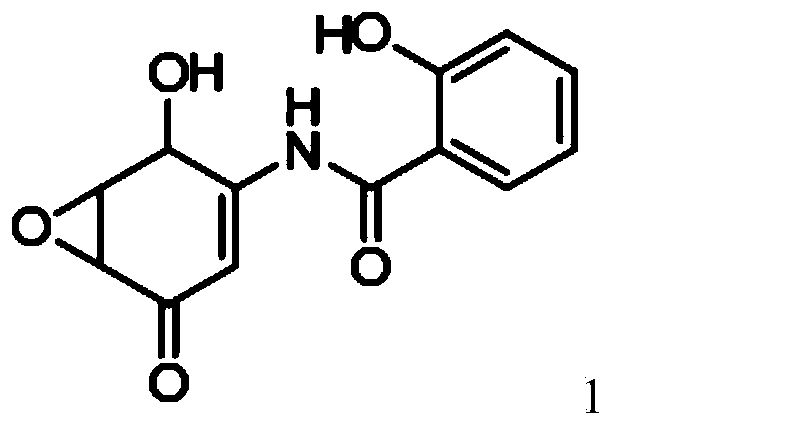
[0083] Example 1: Preparation of compound of formula 1 (5,6-epoxy-4-hydroxyl-3-salicyylamino-2-cyclohexenone, DHM2EQ) crystallization
[0084] Step 1: Synthesis of N-(2-acetoxybenzoyl)-2,5-dimethoxyaniline
[0085]
[0086] 2,5-Dimethoxyaniline (10.0 g, 65.3 mmol) was dissolved in pyridine (100 ml). Under ice-cooling, a solution of O-acetylsalicyloyl chloride (13.0 g, 65.3 mmol) in ethyl acetate (50 ml) was added thereto over 15 minutes, followed by stirring at the same temperature for 15 minutes. After adding water (10ml) to the reaction solution to stop the reaction, add ethyl acetate (500ml), then successively add 3 equivalents of hydrochloric acid (500ml), water (500ml), 2% aqueous sodium bicarbonate (500ml) and water (500ml )washing. The ethyl acetate layer was dried over Glauber's salt, concentrated under reduced pressure and dried in vacuo to obtain the title compound (19.8 g) as a pale yellow syrup. The compound was used directly in the following step witho...
Embodiment 2
[0111] Embodiment 2: preparation formula 1 compound crystallization
[0112] Under heating conditions (40 DEG C), 5g of the product obtained in step 5 of the above embodiment 1 is dissolved in DMF (5 times its weight is the weight of the compound of formula 1), and absolute ethanol (for example, its volume is 5 times that of the compound of formula 1) is added in this solution. 4 times) and glacial acetic acid (the weight of which is 0.3 times that of the compound of formula 1), cooling and crystallization, filtering out the precipitate, washing with absolute ethanol, and vacuum drying to obtain. The crystallization yield was 83.8%.
[0113] The content is 99.7% (HPLC), of which the total impurity is 0.185%, and the largest single impurity is 0.018%.
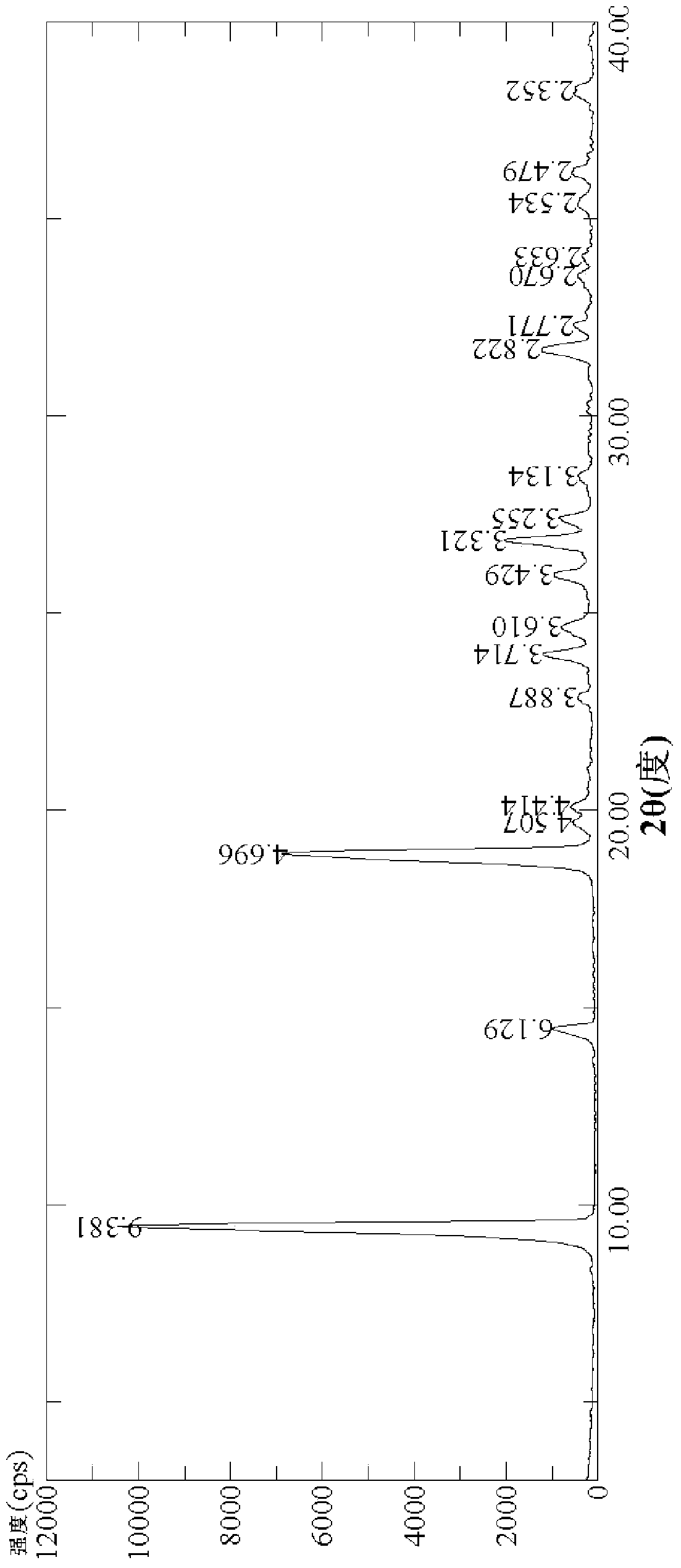
[0114] Determination of powder X-ray diffraction, the results are basically the same as figure 1 Consistent, specifically: with figure 1 The 2θ values of the 19 peaks in the figure 1 The 2θ values of the corresponding ...
Embodiment 3
[0116] Embodiment 3: preparation formula 1 compound crystallization
[0117] Under heating conditions (80 DEG C), 5 g of the product obtained in step 5 of the above embodiment 1 is dissolved in DMF (2 times the weight of the compound of formula 1), and absolute ethanol (for example, its volume is DMF) is added to the solution. 8 times) and glacial acetic acid (its weight is 0.1 times that of the compound of formula 1), cooling and crystallization, filtering out the precipitate, washing with absolute ethanol, and vacuum drying to obtain. The crystallization yield was 85.7%.
[0118] The content is 99.9% (HPLC), of which the total impurity is 0.202%, and the largest single impurity is 0.014%.
[0119] Determination of powder X-ray diffraction, the results are basically the same as figure 1 Consistent, specifically: with figure 1 The 2θ values of the 19 peaks in the figure 1 The 2θ values of the corresponding peaks all differ within ±0.10°.
[0120] A total of four dif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com