Al assistant-modified CUO-ZrO2 water gas shift catalyst and preparation method thereof
A technology for transforming catalysts and water gas, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of active metal copper embedding, inability to fully exert catalytic performance, etc. problem, to achieve the effect of improved catalytic activity and stability, excellent catalytic performance, and enhanced interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
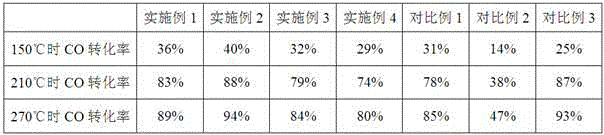
Examples
Embodiment 1
[0018] 1.95g Al(NO 3 ) 3 9H 2 O, 15.08g ZrOCl 2 ·8H 2 O and 12.48g urea were dispersed in 65mL deionized water to prepare a mixed solution [ie n(Al):n(Zr)=10:90, n(Al+Zr):n(urea)=1:4]. The above mixed solution was transferred into a hydrothermal reaction kettle with a volume of 100 mL, the hydrothermal temperature was controlled at 150° C., and the hydrothermal time was 48 hours. The hydrothermal product was washed to remove impurity ions, dried at 120°C for 8 hours, and then calcined at 250°C for 4 hours to obtain Al-modified ZrO 2 carrier. With the aid of ultrasonication, 3 g of ZrO 2 The carrier was dispersed in 200mL of 0.021mol / L Cu(NO 3 ) 2 ·3H 2 O aqueous solution, and then dropwise add 0.5mol / L KOH aqueous solution to the above solution to the terminal pH = 9.0. The obtained product was washed to remove impurity ions, dried at 120°C for 8 hours, and then calcined at 400°C for 4 hours to obtain CuO-ZrO 2 -Al 2 o 3 Water gas shift catalyst.
Embodiment 2
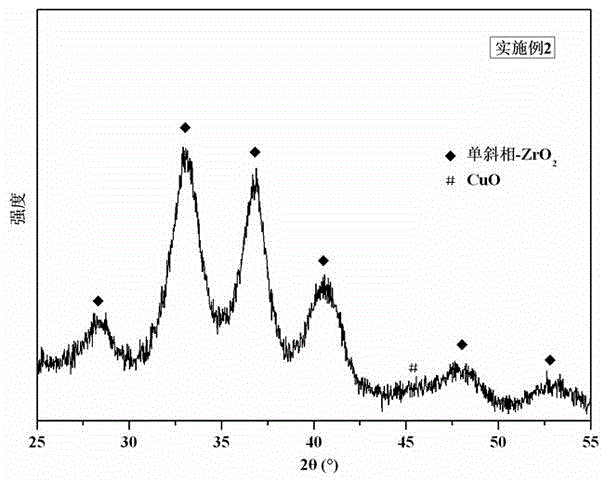
[0020] 0.39g Al(NO 3 ) 3 9H 2 O, 16.41g ZrOCl 2 ·8H 2 O and 6.24g urea were dispersed in 65mL deionized water to prepare a mixed solution [ie n(Al):n(Zr)=2:98, n(Al+Zr):n(urea)=1:2]. The above mixed solution was transferred into a hydrothermal reaction kettle with a volume of 100 mL, the hydrothermal temperature was controlled at 150° C., and the hydrothermal time was 24 hours. The hydrothermal product was washed to remove impurity ions, dried at 120°C for 8 hours, and then calcined at 250°C for 4 hours to obtain Al-modified ZrO 2 carrier. With the aid of ultrasonication, 3 g of ZrO 2 The carrier was dispersed in 200mL of 0.021mol / L Cu(NO 3 ) 2 ·3H 2 O aqueous solution, and then dropwise add 0.5mol / L KOH aqueous solution to the above solution to the terminal pH = 9.0. The obtained product was washed to remove impurity ions, dried at 120°C for 8 hours, and then calcined at 400°C for 4 hours to obtain CuO-ZrO 2 -Al 2 o 3Water gas shift catalyst. figure 1 It is CuO-...
Embodiment 3
[0022] 1.73g Al 2 (SO 4 ) 3 18H 2 O, 21.21g Zr(NO 3 ) 4 ·5H 2 O and 6.24g urea were dispersed in 65mL deionized water to prepare a mixed solution [ie n(Al):n(Zr)=5:95, n(Al+Zr):n(urea)=1:2]. The above mixed solution was transferred into a hydrothermal reaction kettle with a volume of 100 mL, the hydrothermal temperature was controlled at 170° C., and the hydrothermal time was 24 hours. The hydrothermal product was washed to remove impurity ions, dried at 120°C for 8 hours, and then calcined at 250°C for 4 hours to obtain Al-modified ZrO 2 carrier. With the aid of ultrasonication, 3 g of ZrO 2 The carrier was dispersed in 200mL of 0.021mol / L Cu(NO 3 ) 2 ·3H 2 O aqueous solution, and then dropwise add 0.5mol / L KOH aqueous solution to the above solution to the terminal pH = 9.0. The obtained product was washed to remove impurity ions, dried at 120°C for 8 hours, and then calcined at 400°C for 4 hours to obtain CuO-ZrO 2 -Al 2 o 3 Water gas shift catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com