Medicament microsphere and preparation method thereof
A technology of microspheres and drugs, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problem of no celecoxib sustained-release hollow microspheres and immediate-release microspheres, etc., and achieve low cost and high encapsulation efficiency. , good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation: according to the prescription in Table 1, dissolve celecoxib and EC in 20 mL of a mixed solvent of ethyl acetate and ether (3:1) as the dispersed phase; 0.5% poloxamer-188 solution as the continuous phase; Add it into the continuous phase for emulsification under the condition of stirring, raise the temperature to 30°C, stir to remove the organic solvent, filter with suction, wash with distilled water, and dry to obtain celecoxib microspheres.
[0034] Table 1 Preparation of celecoxib microspheres with different EC viscosity formulations
[0035]
[0036] The celecoxib microspheres prepared in Example 1 were evaluated for quality, and the results are shown in Table 2:
[0037] Table 2 Effect of EC viscosity on celecoxib microspheres
[0038]
Embodiment 2
[0040] Preparation: Dissolve celecoxib, release modifier and EC in ethyl acetate and ether (10:
[0041] 1) Use 10mL of the mixed solvent as the dispersed phase; 0.5% poloxamer-188 solution as the continuous phase; add the dispersed phase to the continuous phase for emulsification under stirring conditions, raise the temperature to 30°C, stir to remove the organic solvent, suction filter, Washing with distilled water and drying to obtain celecoxib sustained-release microspheres.
[0042] Table 3 Preparation of different formulations of celecoxib microsphere release modifier
[0043]
[0044]
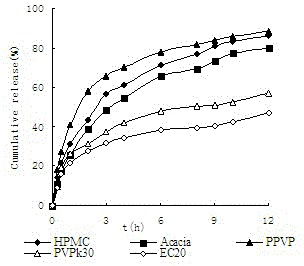
[0045] The celecoxib sustained-release microspheres prepared in Example 2 were evaluated for quality, and the results are shown in Table 4. Among them, the celecoxib sustained-release microspheres prepared with PPVP as the release regulator had the highest encapsulation efficiency.
[0046] Table 4 Effect of release modifiers on celecoxib microspheres
[0047]
[0048] The cel...
Embodiment 3
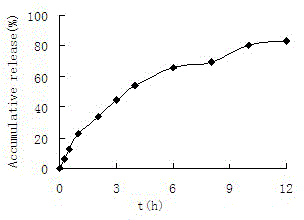
[0050] Dissolve EC20, CXB, and PVPP in 40 mL of a mixed solvent of ethyl acetate and ether (5:1) to make corresponding concentrations of 60 mg·mL -1 , 60mg·mL -1 and 2% solution as the dispersed phase; 0.5% poloxamer-188 solution as the continuous phase; add the dispersed phase to the continuous phase for emulsification under high-speed stirring conditions, raise the temperature to 30°C, stir to remove the organic solvent, filter and wash , dry to get celecoxib microspheres. The quality of the celecoxib microspheres prepared in Example 3 was evaluated, and the results are shown in Table 5.
[0051] Table 5 Effect of poloxamer concentration on celecoxib microspheres
[0052]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| floating rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com