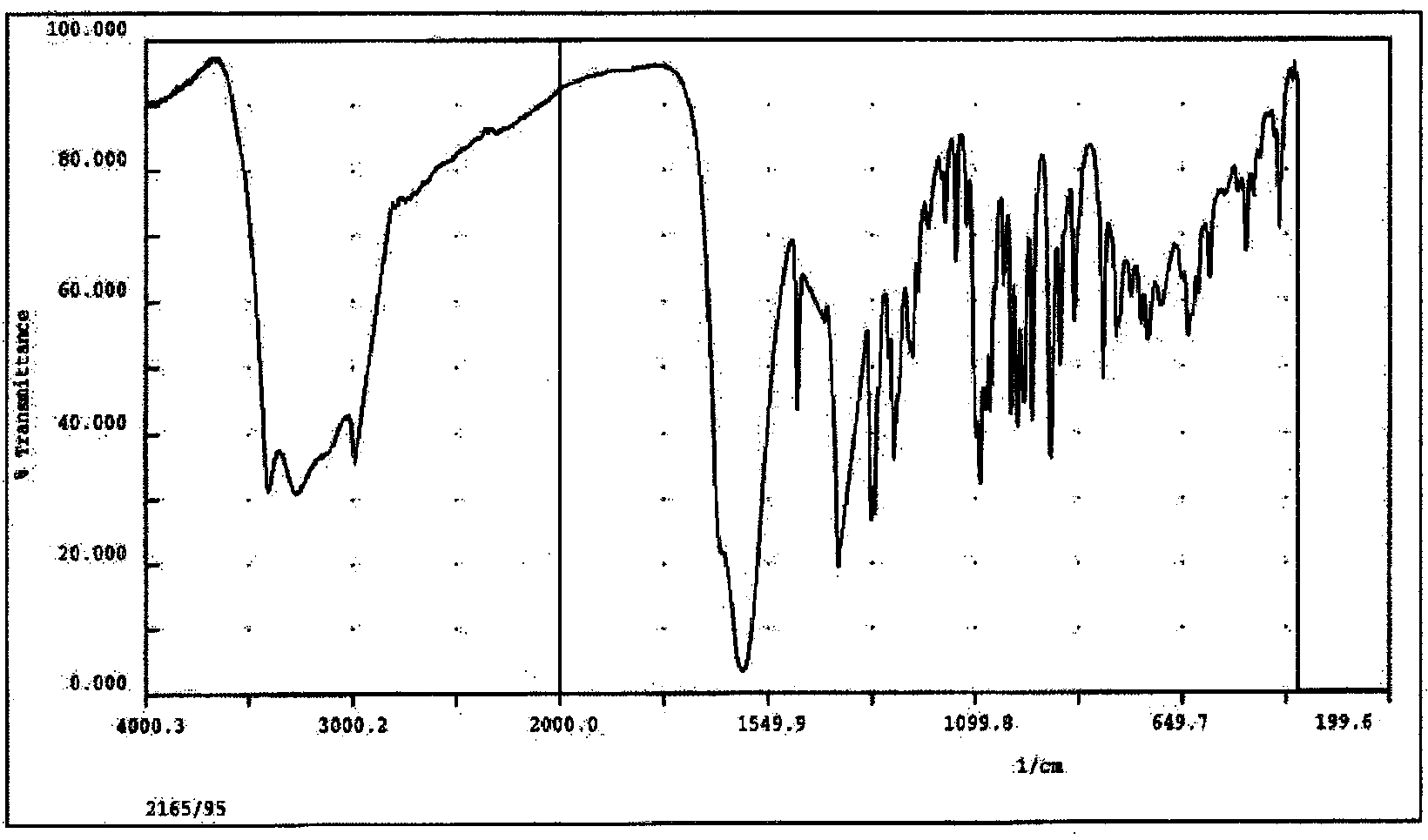
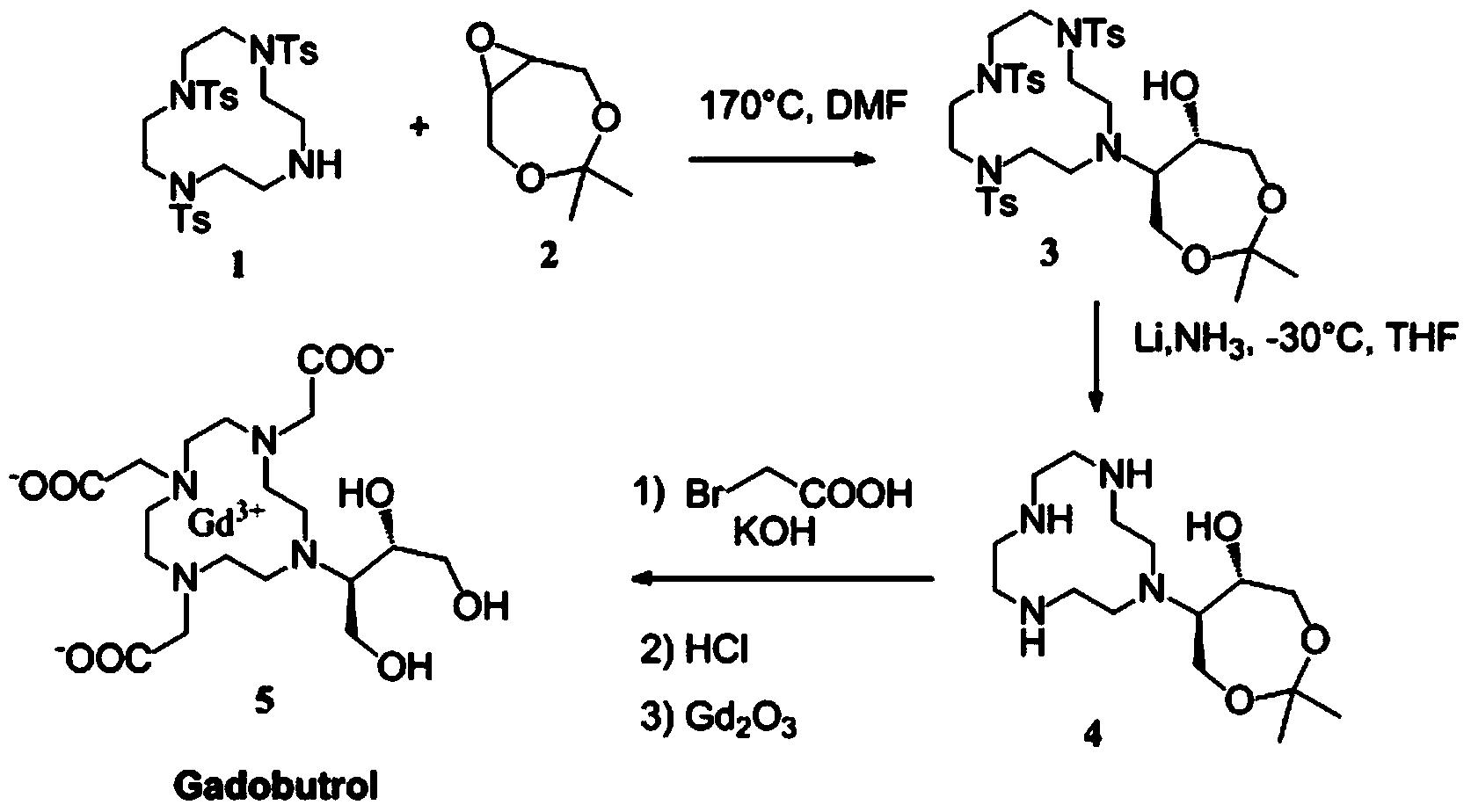
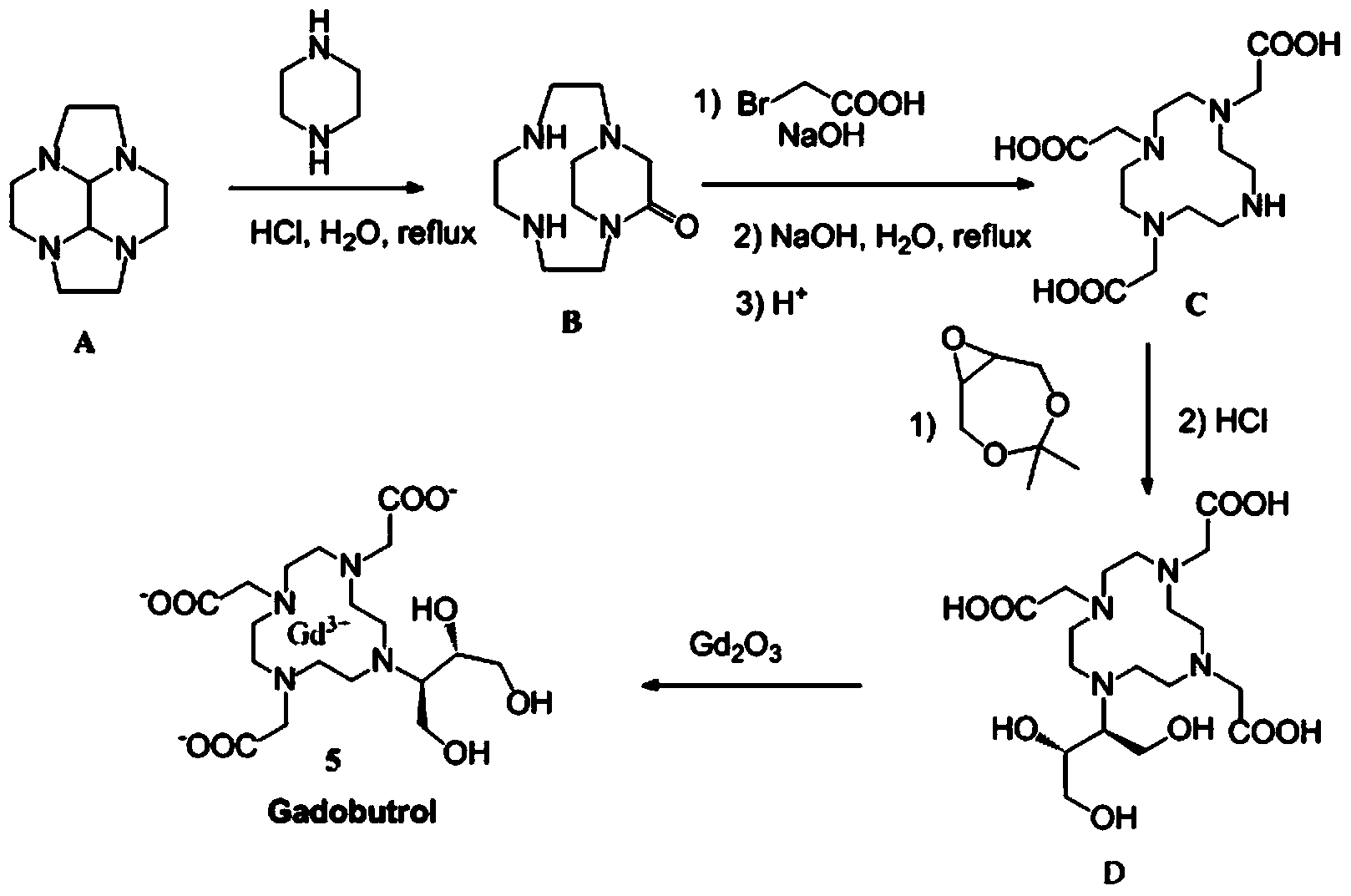
Preparation method for magnetic resonance imaging contrast medium gadobutrol
A technology of magnetic resonance imaging and gadobutrol, applied in organic chemistry and other directions, can solve the problems of harsh acyl hydrolysis conditions and high temperature of ring-opening reaction, and achieve the effects of low equipment requirements, high total yield, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 180.2g (1.0mol) of the compound boron-protected 1,4,7,10-tetraazacyclododecane (I) was dissolved in 800mL of anhydrous tetrahydrofuran, and 45g of NaH (60%, 1.12 mol), warmed up to room temperature and stirred for 0.5h, added 151g (1.05mol) of 4,4-dimethyl-3,5,8-trioxabicyclo[5.1.0]octane (Ⅱ) in batches, React for about 4h (TLC detects that the raw material (I) disappears, and the developing solvent CH 2 Cl 2 :MeOH=10:1), slowly add 300mL of water to the obtained reaction mixture intermediate (Ⅲ), stir for 30min until the intermediate (Ⅳ) is completely hydrolyzed, add 243g (6.0mol) of sodium hydroxide to the mixed system and 311g (3.31mmol) of chloroacetic acid was reacted at 60°C for 6h to obtain intermediate (Ⅴ), the reaction was lowered to room temperature and adjusted to pH 1-1.5 with concentrated hydrochloric acid, then heated and stirred at 50°C for 3h to reach the 4,4 of intermediate (Ⅴ) - The dimethyl ketal group is completely removed, slowly add 200 g (0.55 m...
Embodiment 2
[0040] 180.2 g (1.0 mol) of the boron-protected 1,4,7,10-tetraazacyclododecane (I) was dissolved in 1000 mL of anhydrous tetrahydrofuran, and 45 g of NaH (60%, 1.12 mol), warmed up to room temperature and stirred for 1.0h, then added 155g (1.1mol) of 4,4-dimethyl-3,5,8-trioxabicyclo[5.1.0]octane (II) in batches, at room temperature React for about 4h (TLC detects that the raw material (I) disappears, and the developing solvent CH 2 Cl 2 :MeOH=10:1), slowly add 500mL of water to the obtained reaction mixture intermediate (Ⅲ), stir for 30min until the intermediate (Ⅳ) is completely hydrolyzed, add 243g (6.0mol) of sodium hydroxide to the mixed system and 311g (3.31mol) of chloroacetic acid was reacted at 70°C for 6h to obtain intermediate (Ⅴ), the reaction was lowered to room temperature and adjusted to pH 1-1.5 with concentrated hydrochloric acid, and then heated and stirred at 50°C for 3h to reach 4,4 of intermediate (Ⅴ). - The dimethyl ketal group is completely removed, slo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com