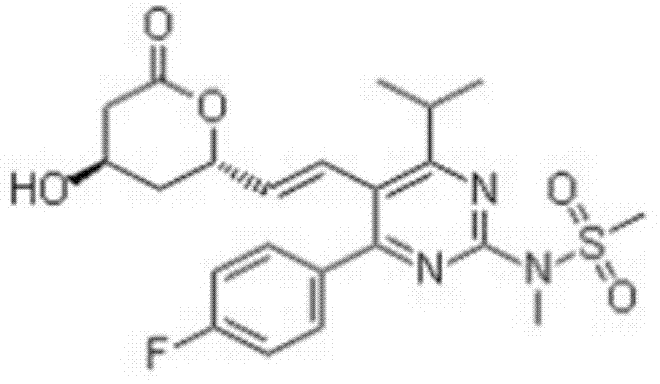
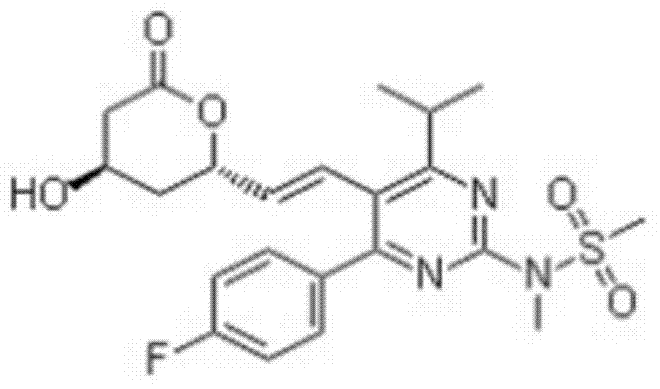
Rosuvastatin lactone
A technology of rosuvastatin lactone and methyl isobutyrylacetate, applied in the field of medicine, can solve problems such as different lipid-lowering properties, and achieve the effects of simple synthesis, strong operability, and simple three-waste treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] The following examples further describe the total synthesis of rosuvastatin lactone.
[0012] 1. Synthesis of 4-(4-fluorophenyl)-6-isopropyl-5-methoxycarbonyl-3,4-2(1H)-dihydropyrimidinone (L)
[0013] Add p-fluorobenzaldehyde (500g), methyl isobutyrylacetate (860g), urea 200g, cuprous chloride, methanol, sulfuric acid into the reaction kettle, and heat to reflux. Cool in an ice-water bath, continue to stir, and then filter with suction. The resulting solid is rinsed with methanol and dried in a drying oven to obtain 1460.17 g of white crystals, with a yield of 98.7%.
[0014] 2. Synthesis of [4-(4-fluorophenyl)-2-hydroxyl-6-isopropyl-5-methoxycarbonylpyrimidine (II)
[0015] Add 65-68% nitric acid and sodium nitrite into the reaction kettle, add intermediate I in batches at 10-25°C, and continue to react at 10-20°C, and the reaction is complete as detected by TLC. Add water to the reaction solution, then add dropwise sodium hydroxide solution to adjust the pH, and pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com