Boracic polyacetylene derivative suitable for fluorinion detection and preparation method thereof
A technology of polyacetylene and fluoride ions, applied in the field of anion detection, to achieve the effects of flexible molecular design, increased sensitivity, and improved bonding efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Synthesis of poly-4-{bis(2,4,6-trimethylphenyl)boronyl}-4'-ethynylbiphenyl
[0033] Synthesis of 4-bromo-4'-trimethylsilylethynylbiphenyl. Take a 250ml dried three-necked flask with a rotor, add 4,4'-dibromobiphenyl under the protection of Ar gas, and pump it three times. Add 50ml THF, 20ml i-Pr through the three-way valve 2 NH, add TMSA after the solid is dissolved, and tighten the three-way valve. Ar gas protection, 45 ℃, overnight reaction. The mixture was spun dry, ether was added, washed first with 1M HCl, then three times with distilled water. The resulting organic phase was dried over anhydrous magnesium sulfate for one hour, filtered and spin-dried. The primary product was separated by silica gel chromatography with petroleum ether as the eluent. 2.35 g of white crystals were obtained (yield 35.4%). 1 H-NMR (400 Hz, CDCl 3 ), δ (ppm): 7.425-7.566 (m, 8H), 0.269 (s, 9H).
[0034] Synthesis of 4-{bis(2,4,6-trimethylphenyl)boronyl}-4'-trimethylsil...
Embodiment 2
[0037] Example 2 Synthesis of poly-9,9-dimethyl-4-{bis(2,4,6-trimethylyl)boronyl}-7-ethynylfluorene
[0038] Synthesis of 9,9-dimethyl-4-{bis(2,4,6-trimethylphenyl)boronyl}-7-ethynylfluorene. This monomer has the same synthesis process as the monomer in Example 1, except that the starting reactant 4,4'-dibromobiphenyl is replaced with 9,9-dimethyl-4,7-dibromobiphenyl Fluorene. White crystals were obtained. 1 H NMR (400MHz, CDCl3)δ7.71–7.47(m,6H), 6.84(s,4H), 3.13(s,1H), 2.32(s,6H), 2.02(s,12H), 1.43(s, 6H). 13 C NMR (100MHz, CDCl3), δ154.9, 153.6, 145.6, 142.2, 142.1, 141.1, 139.7, 138.7, 136.4, 131.5, 131.0, 128.4, 126.8, 121.5, 120.8, 120.0, 84.6, 77.7, 47.0, 21.4. Elemental Analysis (C 35 H 35 B) Calculated value) / %: C: 90.12, H: 7.56, measured value / %: C: 90.15, H: 7.54. The synthesis of the polymer in this example is the same as the synthesis method of the polymer in Example 1. The molecular weight of the polymer is 7.15×10 6 g / mol, molecular weight distributio...
Embodiment 3
[0039] Example 3 Detection of F- by poly-4-{bis(2,4,6-trimethylphenyl)boronyl}-4'-ethynylbiphenyl solution
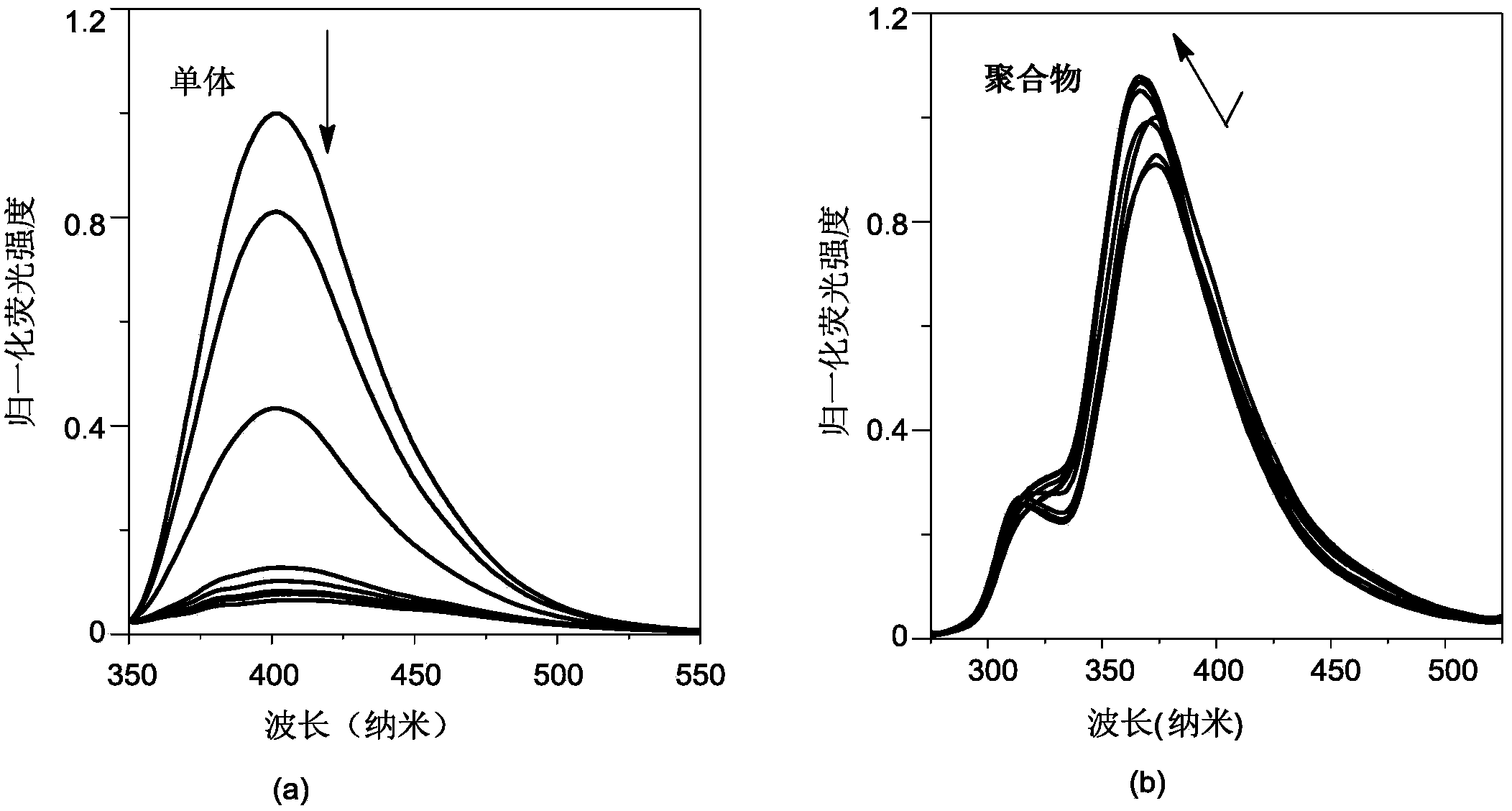
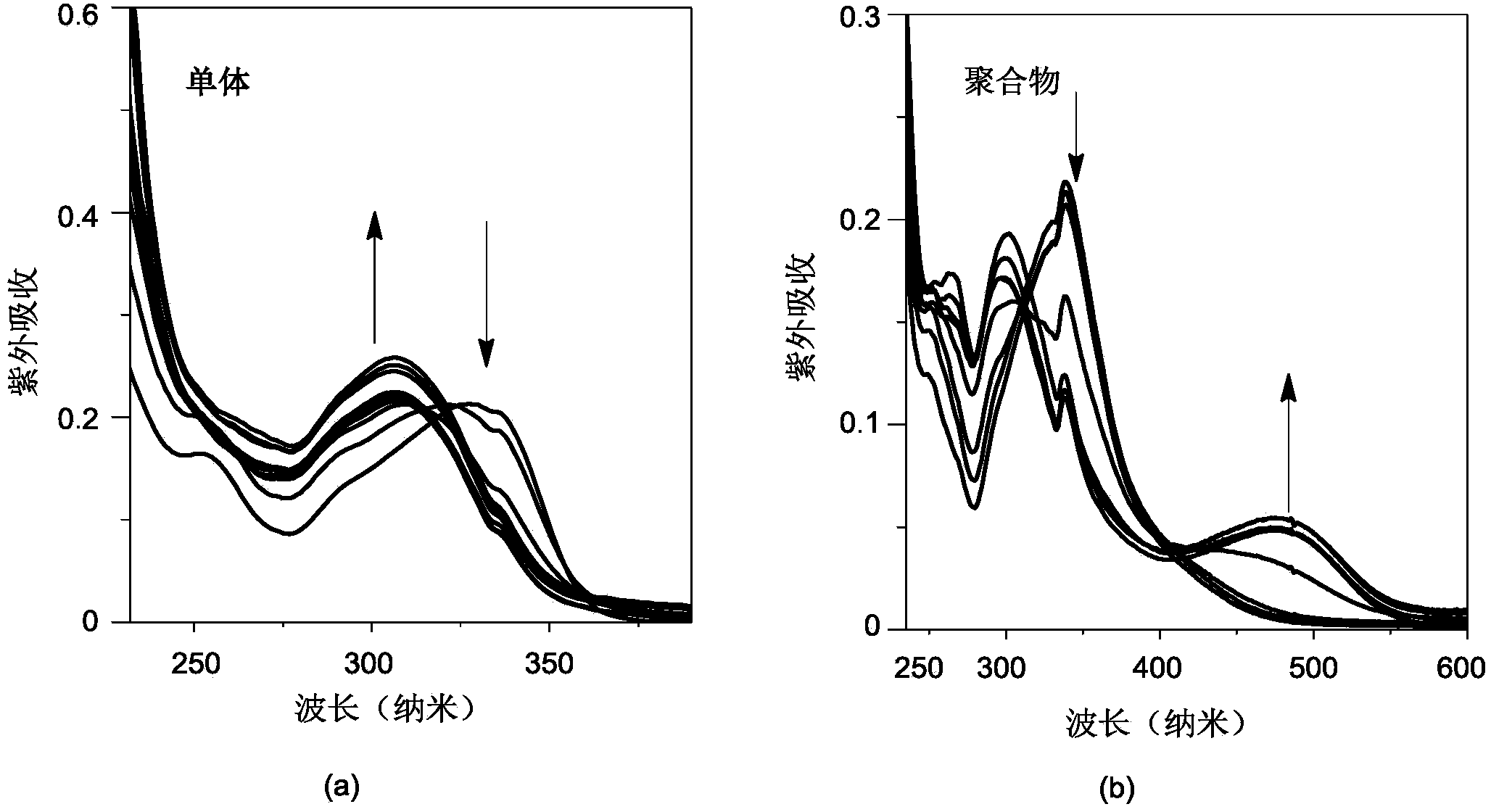
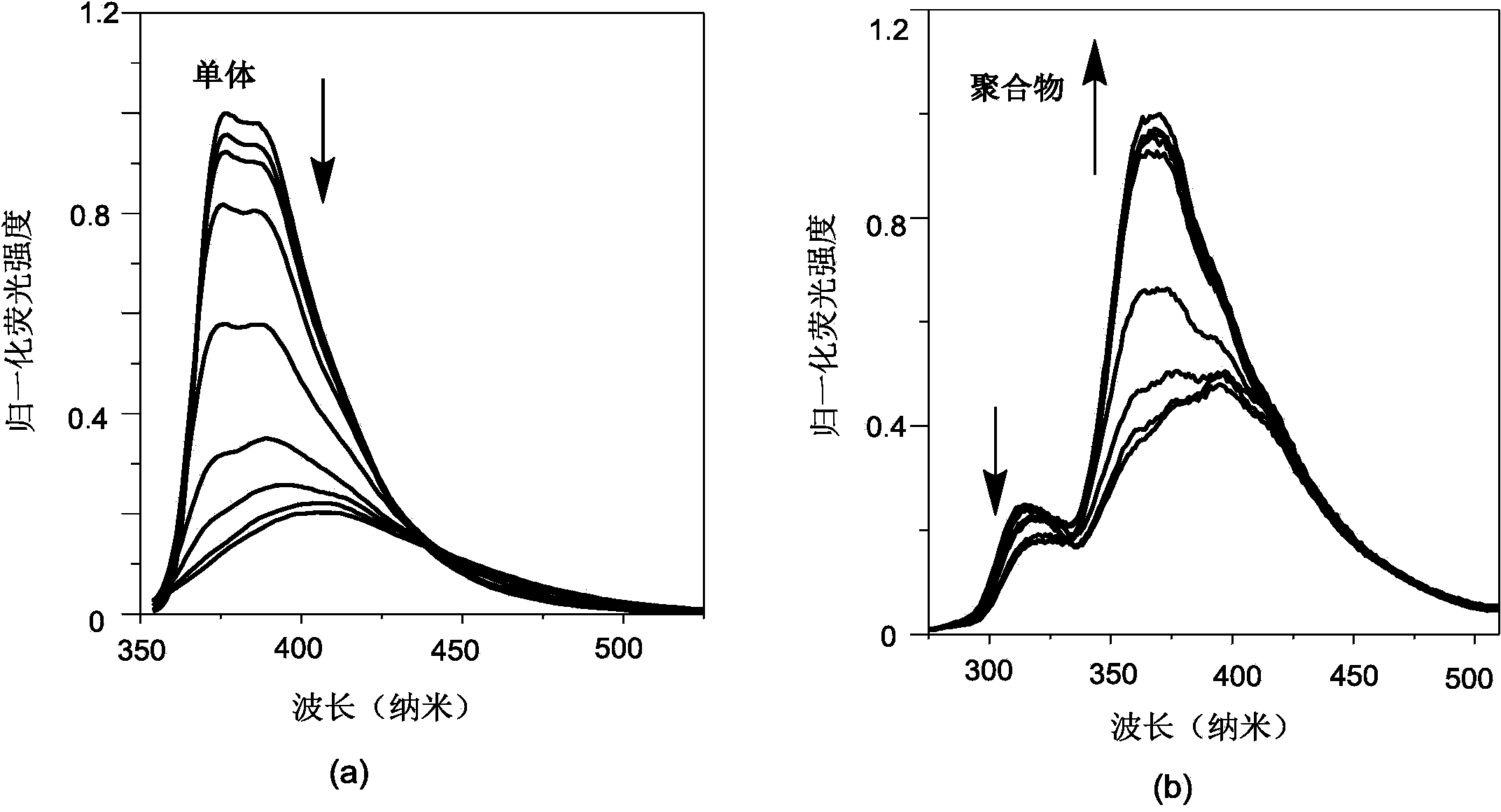
[0040] Configuration concentration 2.9×10 -6 mol / L Bu4NF tetrahydrofuran solution, the concentration prepared in Example 1 is 3.2×10 -6 mol / L polymer poly-4-{bis(2,4,6-trimethylphenyl)boronyl}-4'-ethynylbiphenyl solution and the same concentration of 4-{bis(2, 4,6-Trimethylphenyl)boronyl}-4'-ethynylbiphenyl tetrahydrofuran solution. will Bu 4 NF tetrahydrofuran solution was added dropwise to the solution of monomer and polymer, and the fluorescence change of monomer and polymer after adding fluoride ion was measured by UV-fluorescence photometer. exist figure 1 In the fluorescence spectrum shown, it can be observed that obvious fluorescence quenching phenomenon occurs after the monomer is added dropwise with fluoride ions, while the maximum emission wavelength is blue-shifted after the dropwise addition of fluoride ions to the polymer, but the fluorescence intensity...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com