Method for measuring daily mean stable carbon isotope composition of atmospheric carbon dioxide
A technology for stabilizing carbon isotopes and carbon dioxide, which is applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of difficulty in obtaining stable carbon isotope composition values, measurement errors, etc., and achieves the effect of fewer steps, simple calculation, and faster speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0045] Example of the present invention: it comprises the following steps:
[0046] First, determine the sodium bicarbonate produced by different manufacturers, choose two kinds of δ 13 Sodium bicarbonate whose C value difference is greater than 10‰ is used as a tracer for isotope label 1 and isotope label 2;
[0047] Second, the tracers of isotope label 1 and isotope label 2 were added to the nutrient solution respectively, the concentration of sodium bicarbonate in the nutrient solution was set to 10 mM, the pH was 8.30, and the bicarbonate ion δ in the nutrient solution of isotope label 1 13 C value is δ C1 , bicarbonate ion δ in the nutrient solution labeled with isotope 2 13 C value is δ C2 ;
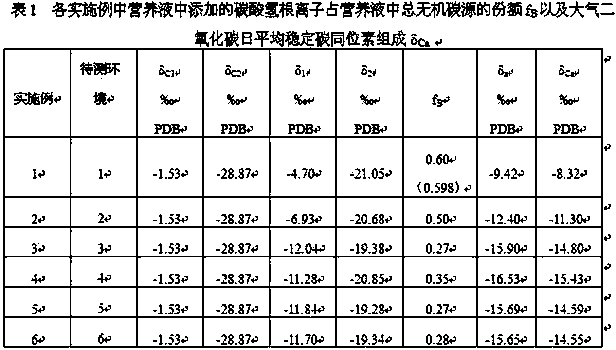
[0048] Thirdly, the above-prepared nutrient solution is used to simultaneously cultivate plants with the same growth cycle in the environment to be tested. After 24 hours of cultivation, the stable carbon isotope composition δ in the two isotope-labeled nutrient solutions is me...
Embodiment 1
[0053] First, determine the sodium bicarbonate produced by different manufacturers, choose two kinds of δ 13 Sodium bicarbonate whose C value difference is greater than 10‰ is used as a tracer for isotope label 1 and isotope label 2;
[0054] Second, the tracers of isotope label 1 and isotope label 2 were added to the Hoagland nutrient solution respectively. The concentration of sodium bicarbonate in the nutrient solution was set at 10 mM, and the pH was 8.30. δ 13 C value is δ C1 , bicarbonate ion δ in the nutrient solution labeled with isotope 2 13 C value is δ C2 ;
[0055] Thirdly, the above-prepared nutrient solution was used to simultaneously cultivate Zhuge Cai with the same growth cycle in the test environment 1. After culturing for 24 hours, the stable carbon isotope composition δ in the two isotope-labeled Hoagland nutrient solutions were measured respectively. 13 C value, denoted as δ 1 and δ 2 value;
[0056] Fourth, the measured δ C1 ,δ C2 ,δ 1 and δ 2...
Embodiment 2
[0060] First, determine the sodium bicarbonate produced by different manufacturers, choose two kinds of δ 13 Sodium bicarbonate whose C value difference is greater than 10‰ is used as a tracer for isotope label 1 and isotope label 2;
[0061] Second, the tracers of isotope label 1 and isotope label 2 were added to the Hoagland nutrient solution respectively. The concentration of sodium bicarbonate in the nutrient solution was set at 10 mM, and the pH was 8.30. δ 13 C value is δ C1 , bicarbonate ion δ in the nutrient solution labeled with isotope 2 13 C value is δ C2 ;
[0062] Thirdly, the above-prepared nutrient solution was used to simultaneously cultivate Brassica napus with the same growth cycle in the test environment 2. After culturing for 24 hours, the stable carbon isotope composition δ in the two isotope-labeled Hoagland nutrient solutions were measured respectively. 13 C value, denoted as δ 1 and δ 2 value;
[0063] Fourth, the measured δ C1 ,δ C2 ,δ 1 and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com