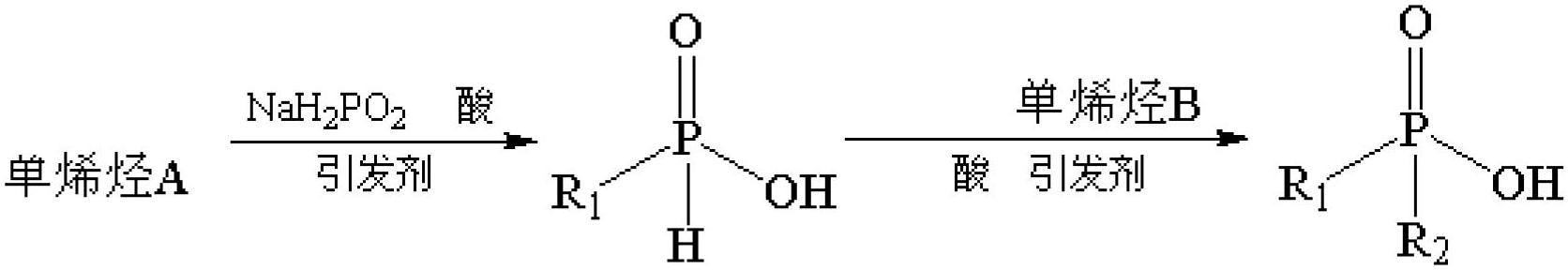
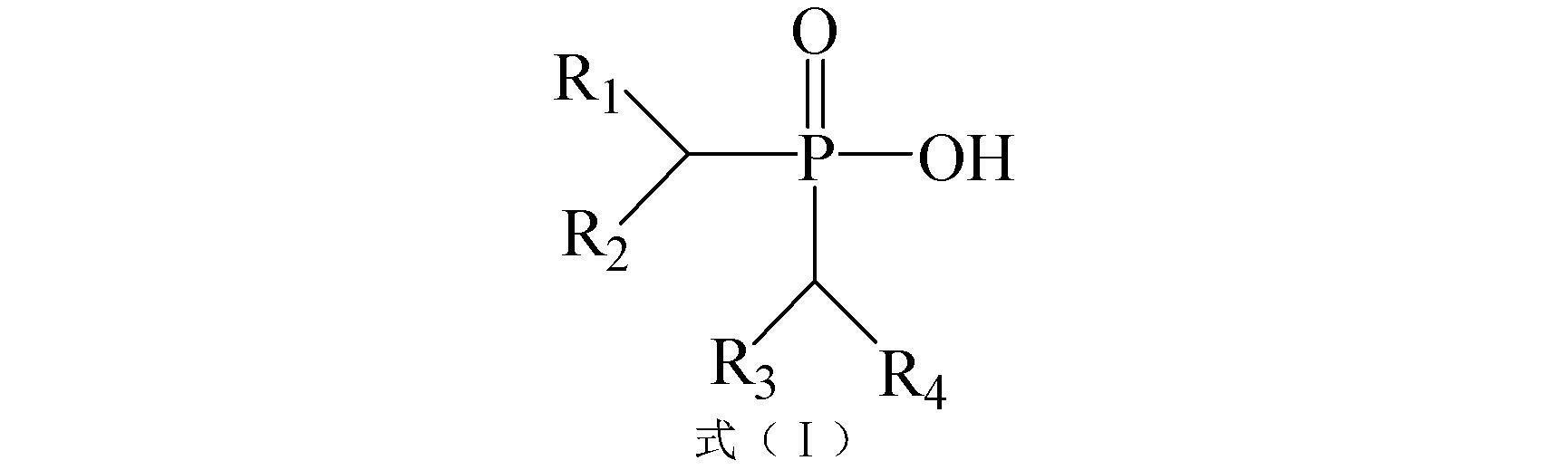
Synthetic method of high-purity asymmetrical dialkyl phosphinic acid
A technology of dialkyl phosphinic acid and monoalkyl phosphinic acid is applied in the field of preparation of organophosphorus compounds, and can solve the problem that extraction and separation of rare earths are still to be studied.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of (n-hexyl)(1-isopropyl-2,2,4,4-tetramethylpentyl)phosphinic acid
[0029] (1) Synthesis of mono(1-isopropyl-2,2,4,4-tetramethylpentyl)phosphinic acid
[0030] according to figure 1 According to the synthetic route shown, 4.05g of dry sodium hypophosphite, 4.12g of glacial acetic acid, 19.14g of triisobutylene, and 0.37g of di-tert-butyl peroxide (DTBP) were weighed sequentially in a stainless steel airtight container lined with 40ml of polytetrafluoroethylene. In the reaction kettle, start magnetic stirring, and react at 135° C. for 15 hours. Cool down to room temperature, add 0.35g of DTBP, and react at 135°C for another 15h. After cooling down to room temperature, 0.35 g of DTBP was added, and the reaction was continued at 135° C. for 15 h. Dilute the product with 50ml of anhydrous ether and transfer it to a 250ml separatory funnel, wash twice with 30ml and 20ml of deionized water, add 30ml of 4% NaOH solution to shake, let stand, the solution is divid...
Embodiment 2
[0034] Synthesis of (3,3-dimethylbutyl)(1-isopropyl-2,2,4,4-tetramethylpentyl)phosphinic acid
[0035] according to figure 1 According to the synthetic route shown, weigh 4.72g of the above-mentioned mono(1-isopropyl-2,2,4,4-tetramethylpentyl)phosphinic acid, 4.35g of 3,3-dimethyl-1-butene g, 2.57 g of propionic acid, and 0.38 g of azobisisobutyronitrile (AIBN) were placed in a stainless steel airtight reaction kettle lined with 20 ml of polytetrafluoroethylene, magnetic stirring was started, and the reaction was carried out at 90 ° C for 15 h. Cool down to room temperature, add 0.33g of AIBN, react at 90°C for another 15h, and cool down to room temperature. Add 0.38g of AIBN, react at 90°C for 15h, and cool down to room temperature. The product was diluted with 30ml n-hexane, washed twice with deionized water (20ml*2), washed twice with 4% NaOH solution (30ml*2), 10% H 2 SO 4 Acidify and wash twice (20ml*2). The n-hexane phase was washed three times with saturated brine...
Embodiment 3
[0037] Synthesis of (cyclooctyl)(1-decyl)phosphinic acid
[0038] (1) Synthesis of monocyclic octylphosphinic acid
[0039] according to figure 1 According to the synthetic route shown, 4.05g of dry sodium hypophosphite, 5ml of concentrated hydrochloric acid, 15.91g of cyclooctene, 0.22g of DTBP, and 0.11g of benzoyl peroxide (BPO) were weighed sequentially in 40ml of polytetrafluoroethylene. In a lined stainless steel airtight reaction kettle, start magnetic stirring, and react at 135°C for 15h. Cool down to room temperature, add DTBP 0.25g, BPO 0.08g, and react at 135°C for 15h. After cooling down to room temperature, 0.26 g of DTBP and 0.10 g of BPO were added, and the reaction was continued at 135° C. for 15 h. Dilute the product with 50ml of cyclohexane and transfer it to a 250ml separatory funnel, wash twice with 30ml and 20ml of deionized water, add 30ml of 4% NaOH solution for shaking, let it stand, and discard the organic phase. Then add 20ml of diethyl ether to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com