Blood stasis removing capsule containing Salvia miltiorrhiza and Radix Astragali, and preparation method thereof
A technology for capsules and stasis removal, which is applied in the directions of capsule delivery, pharmaceutical formulations, and medical preparations with non-active ingredients, etc. The effect of body characteristics and content stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Step 1: Dry and pulverize 75g of scorpion, 45g of curcuma, 75g of ground beetle and 75g of corydalis into fine powder, pass through a 100-mesh sieve for later use;
[0016] Step 2: 150g of astragalus, 150g of salvia miltiorrhiza, 75g of codonopsis pilosula, 75g of Chinese yam, 100g of Smilax smilax, 75g of angelica, 150g of spatholobus, 75g of Gorgon, 150g of Houttuynia cordata, 45g of trigonum, 150g of Patrinia, cinnamon 15g, 45g Atractylodes Rhizome, 22.5g Pao Jiang, 45g Toosendan and 75g Sophora flavescens, add water and decoct three times, add 10 times the amount of water to extract for 3 hours, add 8 times the amount of water to extract for 2 hours, add 8 times the amount of water to extract for 2 hours, add 8 times the amount of water to extract Extract with water for 1 hour, filter, combine the filtrates, concentrate to an extract with a relative density of 1.28-1.30 at 60°C;
[0017] Step 3: Mix the fine powder and extract evenly, dry, and crush into fine powder...
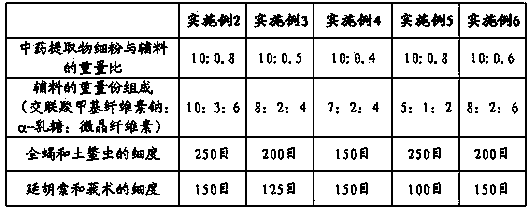
Embodiment 2-6
[0019] Except the following table parameter, other preparation processes are with embodiment 1, specifically see the following table:
[0020]
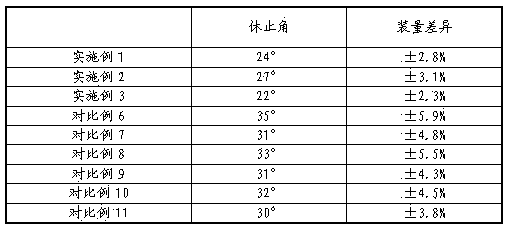
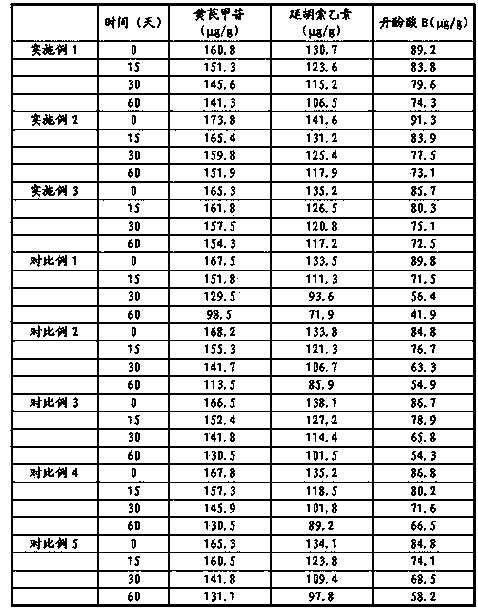
Embodiment 7
[0021] Example 7 Stability Investigation of Danhuang Quyu Capsules Prepared in Examples 1-3
[0022] According to the stability guidelines of the second appendix of the Chinese Pharmacopoeia 2010 edition, the stability of the relevant samples was investigated, and the stability of the drug was investigated at 25°C for 36 months, and at 25°C and a relative humidity of 75% for 60 days. The contents of astragaloside IV, tetrahydropalmatine and salvianolic acid B in the capsules were determined by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com