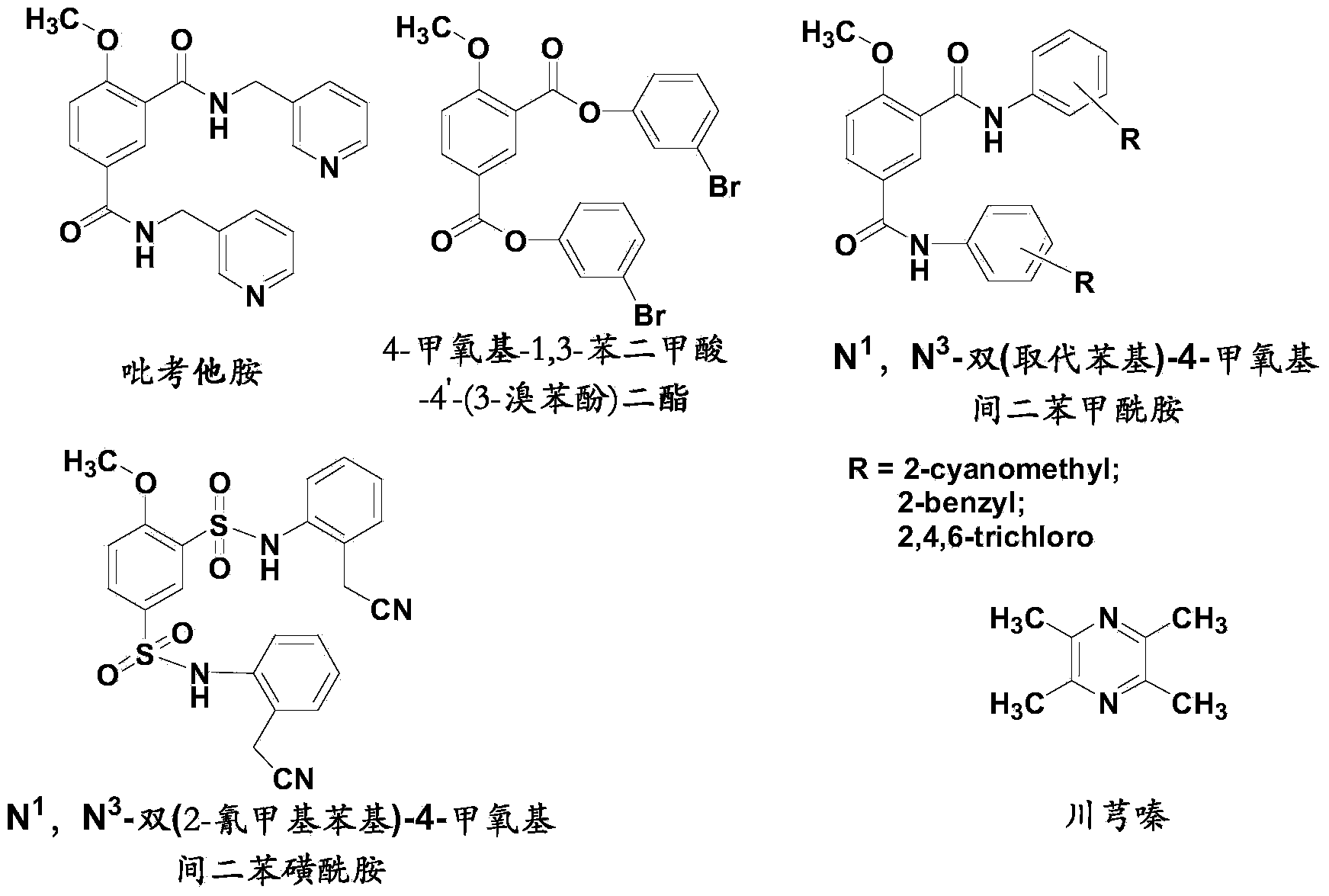
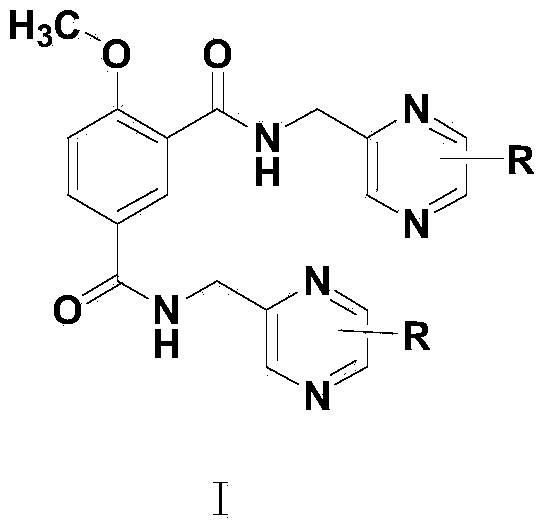
Picotamide analogues as well as preparation method and application thereof
A technology of picotamide and its analogues, applied in the field of medicinal chemistry, can solve the problems of unreported drug efficacy and activity of compounds, and achieve the effect of treating thromboembolic diseases and inhibiting platelet aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
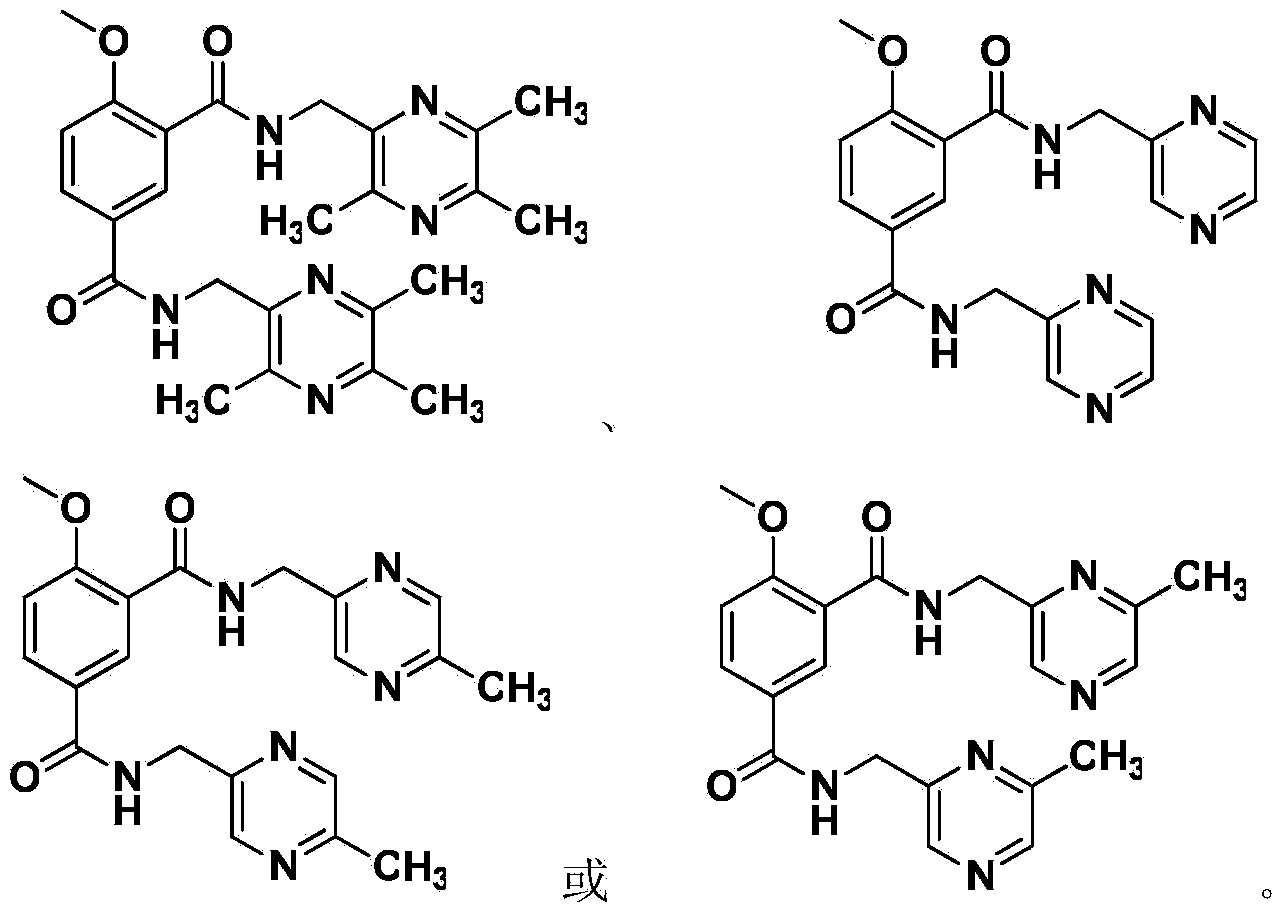
[0031] 4-methoxy-N 1 , N 3 -Synthesis of bis(3,5,6-trimethylpyrazin-2-yl)methyl)isophthalamide (PKL1)
[0032] 1.1 Synthesis of 2-bromomethyl-3,5,6-trimethylpyrazine
[0033]
[0034] In a 250ml three-necked bottle, add raw materials anhydrous ligustrazine (20g, 0.147mol), NBS (26.75g, 0.15mol), benzoyl peroxide (0.05g, 0.0002mol), solvent CCl 4 (75mL), the solution was orange-yellow turbid, irradiated by an incandescent lamp, the oil bath was heated to 75°C, and refluxed for 10 hours. TLC [V (petroleum ether): V (ethyl acetate) = 2:1 as developing agent] showed The reaction is complete (raw material Rf=0.4, product Rf=0.6), the reaction solution is purple, and the generated succinimide is removed by filtration to obtain a purple filtrate, and CCl is recovered under reduced pressure 4 The final purple-red viscous liquid was distilled under reduced pressure, and the product was collected at 99-101°C / 2mmHg fraction to obtain 20 g of 2-bromomethyl-3,5,6-trimethylpyrazine co...
Embodiment 2
[0046] 4-methoxy-N 1 , N 3 -Synthesis of bis(5-methylpyrazin-2-yl)methyl)isophthalamide (PKL2)
[0047] 2.1 Synthesis of 2-chloromethyl-5-methylpyrazine
[0048]
[0049] Add 2,5-dimethylpyrazine (6g, 55.6mmol) and carbon tetrachloride (50mL) successively in a three-necked flask, stir and dissolve and add N-chlorosuccinimide (NCS) (7.4g, 55.6mmol) and benzoyl peroxide (0.05g, 0.21mmol), the reaction mixture was stirred under incandescent light, and refluxed for about 18h, TLC [V (ethyl acetate): V (petroleum ether) = 1:2 As a developing agent] After detecting that the reaction is basically complete, the reaction solution is cooled to 0°C, and after standing for 1 hour, it is filtered, the filter cake is washed with cold carbon tetrachloride, the filtrate and washing liquid are combined, and the carbon tetrachloride is evaporated under reduced pressure. A light brown oil was obtained, which was dissolved in dichloromethane and decolorized by activated carbon to obtain a l...
Embodiment 3
[0060] 4-methoxy-N 1 , N 3 -Synthesis of bis(pyrazin-2-yl)methyl)isophthalamide (PKL3)
[0061]
[0062] (Pyrazin-2-yl) methylamine is operated according to embodiment 2.1, 2.2, 2.3, 4-methoxy-N 1 , N 3 -The synthesis of two (pyrazine-2-yl) methyl) isophthalamides is operated according to Example 2.4 to obtain PKL 3 White crystals, yield 28.6%, m.p.176.4-178.2°C. 1 H NMR (CDCl 3 ,400MHz)δ:8.83(brs,1H,NH),8.69(s,1H,pyrazine ring-H),8.67(s,1H,pyrazine ring-H),8.65(s,1H,pyrazine ring-H) H),8.55(s,1H,pyrazine ring-H),8.53(s,1H,pyrazine ring-H),8.52(s,1H,pyrazine ring-H),8.51(s,1H,ArH) ,8.18(d,J=8.8Hz,1H,ArH),7.45(brs,1H,NH),7.12(d,J=8.8Hz,1H,ArH),4.86(d,J=5.2Hz,2H,- NHCH 2 -),4.83(d,J=5.2Hz,2H,-NHCH 2 -),4.08(s,3H,CH 3 O-); 13 C NMR (CDCl 3 ,100MHz) δ:166.17,164.74,160.12,152.86,152.51,144.14,144.07,143.90,143.87,143.52,143.45,133.67,130.18,126.84,120.47,111.89,52K18,Br(IR -1 )υ: 3360, 3080, 2929, 1673, 1599, 1661, 1490, 1408, 1276, 843, 760, 617; ESI-MS for C 19 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com