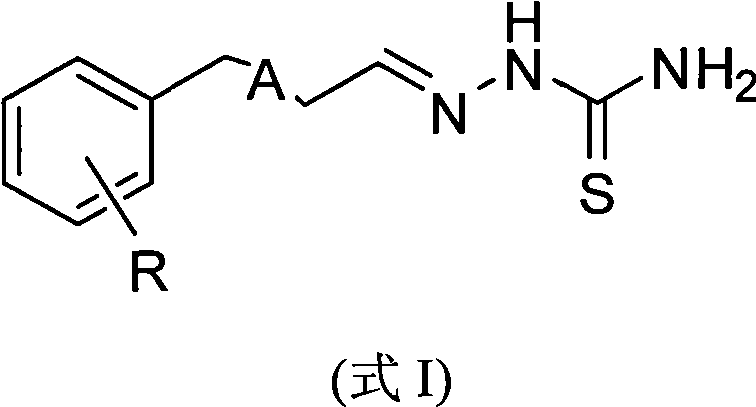
Novel substituted phenylpropionaldehyde (benzylideneacetaldehyde) thiosemicarbazone compounds and applications of the compounds as agricultural bactericides
A compound, multi-substituted technology, used in fungicides, applications, biocides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
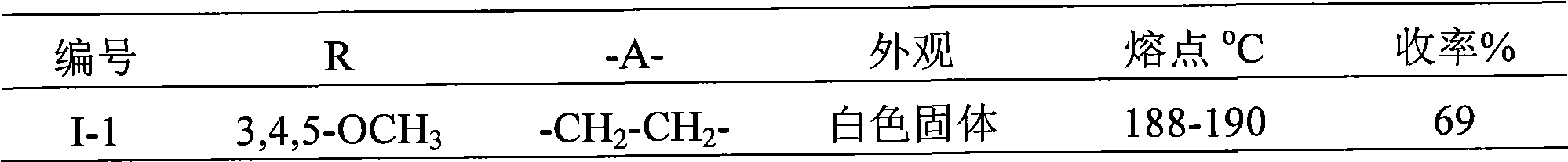
[0013] Embodiment 1: the preparation of 4-bromophenylacrylaldehyde thiosemicarbazone (I-5)
[0014] Add 1.06g (5mmol) 4-bromophenylacrolein, 25mL organic solvent ethanol and 0.41g (4.5mmol) thiosemicarbazide to a 50mL three-neck flask, add 3.0mg (0.05mmol) acidic compound acetic acid in one batch, and heat to 78°C The condensation reaction was carried out under reflux for 1 hour, and the reaction was stopped. The reaction solution was spin-dried and recrystallized from ethanol to obtain 0.98 g of a yellow solid, yield: 77%. The appearance and fusing point of this yellow solid product are shown in Table 1, and its 1 H NMR spectrum data are shown in Table 2. As can be seen from Table 2, the structure of the product is correct.
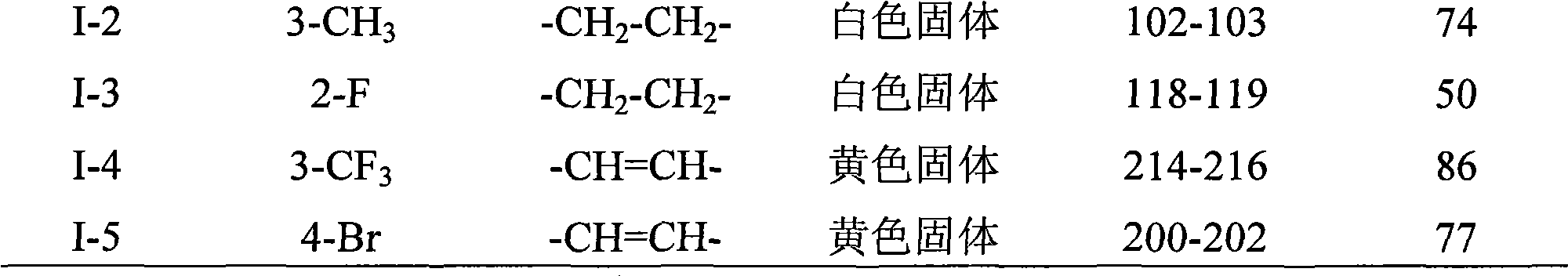
[0015] According to the same method as above for the preparation of compound I-5, only the compound shown in formula I is replaced according to the R group shown in Table 1 to obtain the corresponding products I-1, I-2, I-3 and I-4 , the appearance, ...
Embodiment 2
[0021] Embodiment 2: the inhibitory activity of formula I compound to 4 kinds of plant pathogenic bacteria
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com