Composition containing nimodipine, preparation method and application thereof
A technology of nimodipine and a composition is applied in the field of nimodipine-containing composition and preparation thereof, which can solve the problems of high drug safety risk, large toxic and side effects, poor stability and the like, and achieves simplified process flow and high drug loading capacity. , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The embodiment of the present invention provides a preparation method of a composition containing nimodipine, comprising:
[0042] Take water for injection, adjust the pH of the water for injection to be 8-12, and form an aqueous phase;
[0043] Take the surfactant and add it to the water phase, under nitrogen protection, under the condition of water temperature of 50-70°C, shear the surfactant until it is uniformly dispersed;
[0044] Adding nimodipine into the above-mentioned solution containing surfactant, shearing to obtain a suspension;
[0045] Homogenize the suspension at 40°C to 65°C to obtain a nanosuspension with a particle size of less than 200nm (nanometer);
[0046] The nanosuspension was sterilized by circulating steam under the conditions of 121° C. and F0≧12.
[0047] In the preparation method of the composition containing nimodipine, there is no need to adopt airflow pulverization or precipitation with organic solvent and remove the organic solvent, onl...
Embodiment 1
[0056] Take 1000L of water for injection, and adjust the pH of the water for injection to 8-12 with 2% sodium hydroxide solution to form an aqueous phase;
[0057] Weigh the amount of surfactant (egg yolk lecithin) shown in Table 1, the content of which is the mass concentration, add it to the water phase, under the protection of nitrogen, cut the surfactant until it is evenly dispersed, and control the water temperature at 50-70 °C;
[0058] The nimodipine (content is mass concentration) of the amount shown in Table 1 is added in the above-mentioned solution containing surfactant, and sheared to form a suspension;
[0059] Homogenize the suspension in the way of gradient boosting: first carry out 300-500bar (bar) low-pressure circulation treatment, and then perform 900-1100bar high-pressure circulation treatment to prepare a nano-suspension with a particle size of less than 200nm. The homogenization temperature Controlled at 40~65℃;
[0060] The nanosuspension is sterilized ...
Embodiment 2~15
[0063] Each component and content etc. of preparation composition of embodiment 2~15 are as shown in table 1, and embodiment 2~15 except nimodipine content, surfactant content and aqueous phase pH and embodiment 1 are different, all the other and implementation Example 1 is the same.
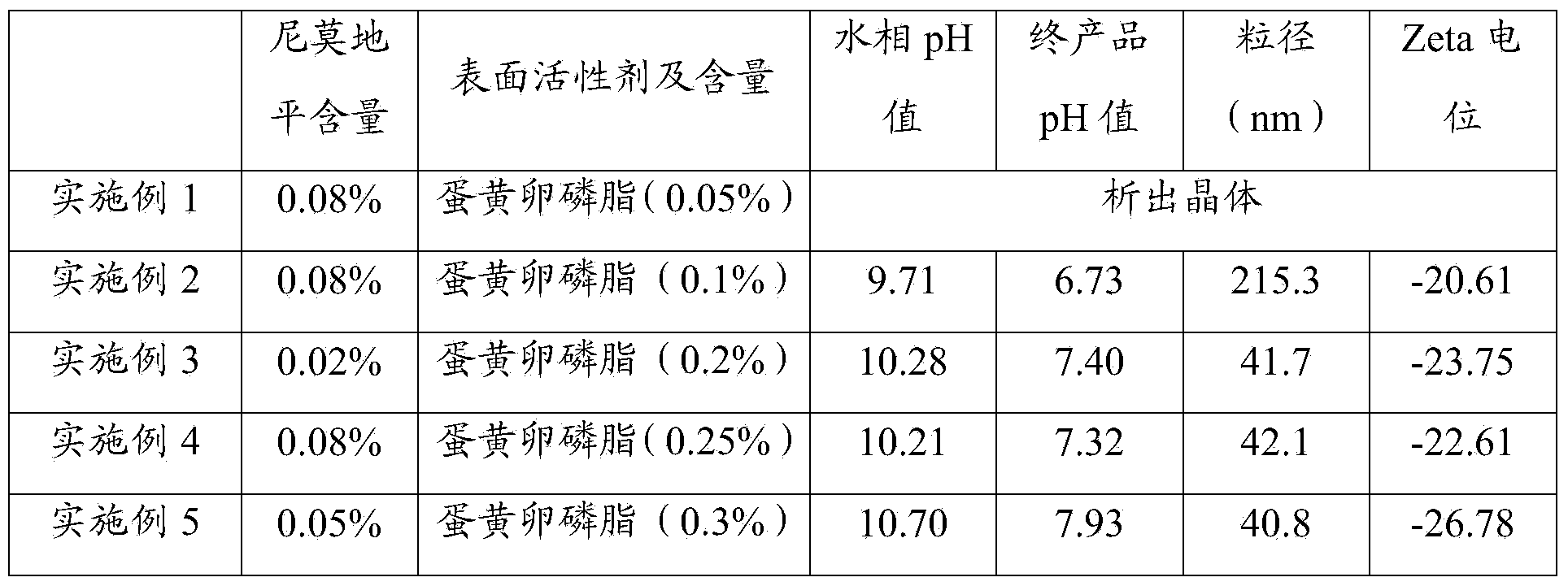
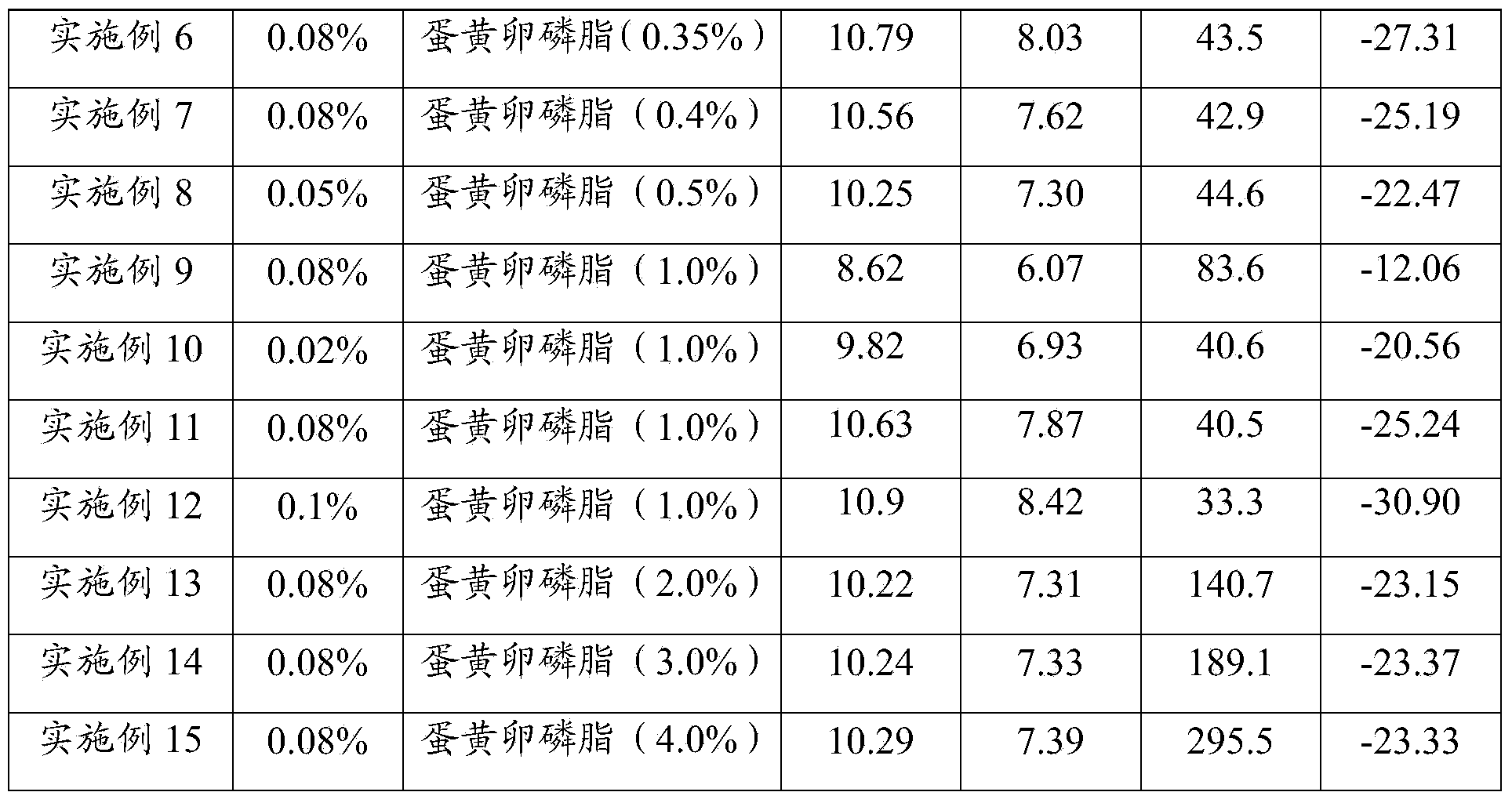
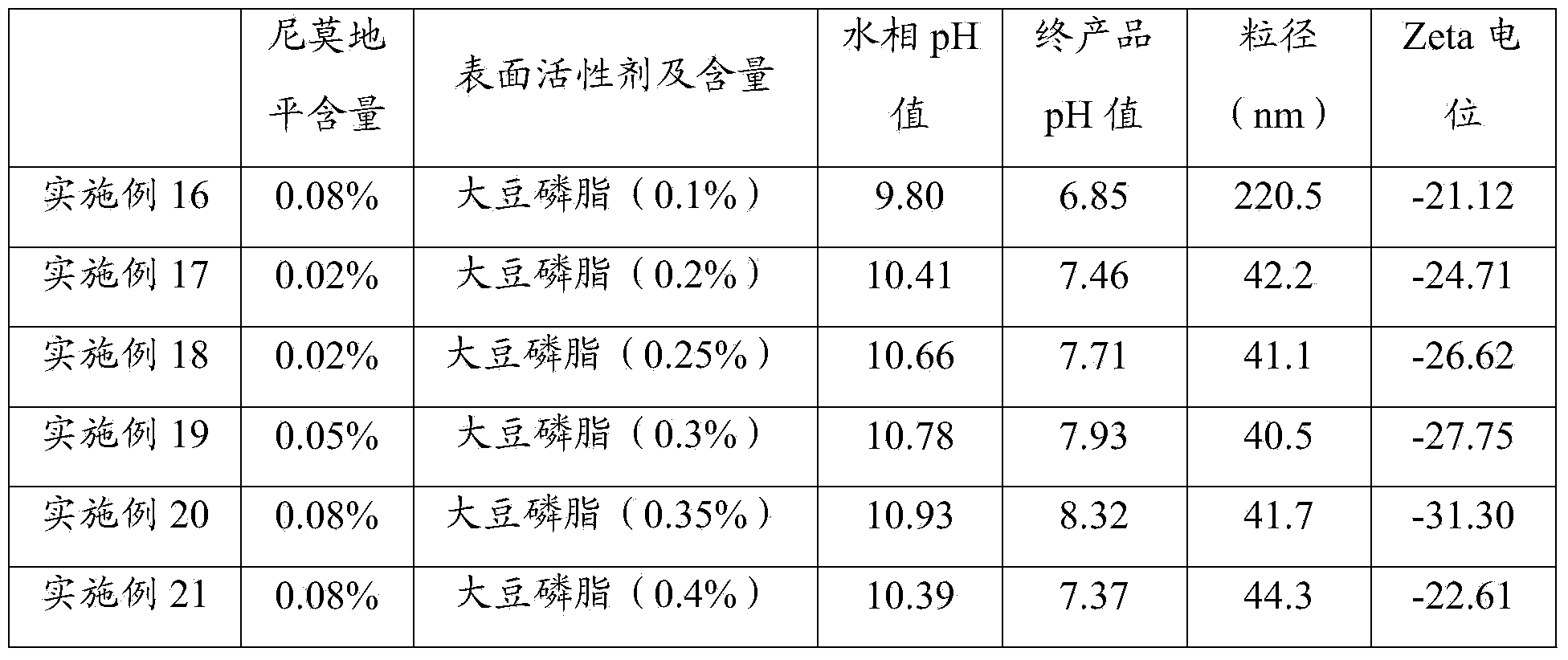
[0064] Table 1 The components used in Examples 1 to 15 and the test result table
[0065]
[0066]
[0067] It can be seen from Table 1 that when egg yolk lecithin is used as a surfactant, and the content is 0.2% to 3%, especially at 0.2% to 1%, the particle size of the prepared nimodipine nanosuspension injection is small and uniform , the pH value of the final product is 6.5~8.5, especially when the pH value is 7.5~8.5, the absolute value of Zata potential is greater than 25mV, even for embodiment 12, Zata potential is greater than 30mV, illustrates that the nimodipine nano-mixture prepared by the present invention Suspension injections are more stable.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com