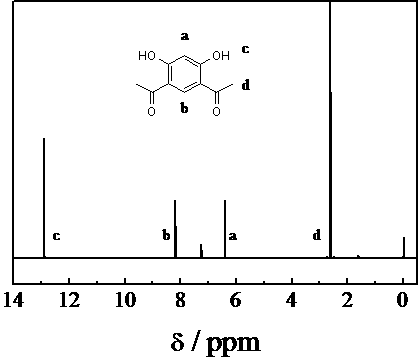
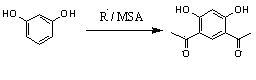
Method for preparing 4,6-diacetylresorcinol by acetylating resorcinol
A technology of diacetyl resorcinol and resorcinol acetylation is applied in the field of preparation of 4,6-diacetyl resorcinol, and can solve the problems of high cost, environmental pollution, difficult operation and the like, and achieves the The effect of low viscosity, reducing chemical pollution and improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] temperature 90℃ , MSA / AN / Resorcinol (molar ratio) = 2.2 / 2.2 / 1, acetylation of resorcinol to prepare 4,6-diacetyl resorcinol: Measure 7mL of methanesulfonic acid and 11mL of acetic anhydride in 100mL In the three-necked flask, add 5.5g resorcinol into it, stir to dissolve, then raise the temperature to 90°C, take samples for HPLC every half hour, observe the reaction, after the reaction is completed, cool to room temperature, add water 15mL and methanol 10mL, heated to 80°C and stirred for half an hour, cooled to room temperature in the air, and filtered with suction to obtain 4,6-diacetylresorcinol with a conversion rate of 77.8%.
Embodiment 2
[0014] Temperature 110°C, MSA / AN / Resorcinol (molar ratio) = 2.2 / 2.2 / 1, acetylation of resorcinol to prepare 4,6-diacetylresorcinol: Measure 7mL of methanesulfonic acid and 11mL of acetic anhydride in In a 100mL three-neck flask, under stirring conditions, add 5.5g resorcinol into it, stir to dissolve, raise the temperature to 110°C, take samples for HPLC every half hour, observe the reaction, after the reaction is completed, cool to room temperature, add water 15mL and methanol 10mL, heated to 80°C and stirred for half an hour, cooled to room temperature in the air, and suction filtered to obtain 4,6-diacetylresorcinol with a conversion rate of 84.6%.
Embodiment 3
[0016] Temperature 130°C, MSA / AN / Resorcinol (molar ratio) = 2.2 / 2.2 / 1, acetylation of resorcinol to prepare 4,6-diacetylresorcinol: Measure 7mL of methanesulfonic acid and 11mL of acetic anhydride in In a 100mL three-neck flask, under stirring conditions, add 5.5g resorcinol into it, stir to dissolve, raise the temperature to 130°C, take samples for HPLC every half hour, observe the reaction, after the reaction is completed, cool to room temperature, add water 15mL and 10mL of methanol, heated to 80°C and stirred for half an hour, cooled to room temperature in air, and suction filtered to obtain 4,6-diacetylresorcinol with a conversion rate of 90.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com