Method for synthesizing perfluoropolyether from perfluoropolyether peroxide
A technology of perfluoropolyether peroxide and perfluoropolyether, which is applied in the field of organic chemistry, can solve the problems of reducing the production efficiency of perfluoropolyether, the difficulty of handling small molecules, and increasing the production cost, etc., and achieve the refinement of product application fields , Good economic value, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Perfluoropolyether peroxide prepared by photooxidative polymerization: Rf-O-O-Rf, wherein Rf can be a mixture of any possible situation in the following formula:
[0033] A-O-(-C(T f ) (OCF 3 ) CF 2 -O) m (G f -O-O) n -(CF 2 O) p -(CF 2 OO) q -.
[0034] Self-made 5L stainless steel reaction kettle, built-in stirring, cooling coil, ultraviolet lamp, the measured peroxide value is 9.2×10 -2 Add 2kg of perfluoropolyether peroxide prepared by mmol / g photooxidative polymerization and 1.2kg of trifluorotrichloroethane into the reaction kettle, turn on the ultraviolet lamp (wavelength 365nm), when the temperature rises to 80°C, Slowly feed hexafluoropropylene intermittently, the temperature is controlled at about 100 ° C ~ 105 ° C, the pressure is 1 MPa, a total of 40 g of hexafluoro propylene is added, and 20 g is introduced per hour. After passing through the hexafluoro propylene, react for another 3 hours, then cool down and discharge . The peroxide value of th...
Embodiment 2
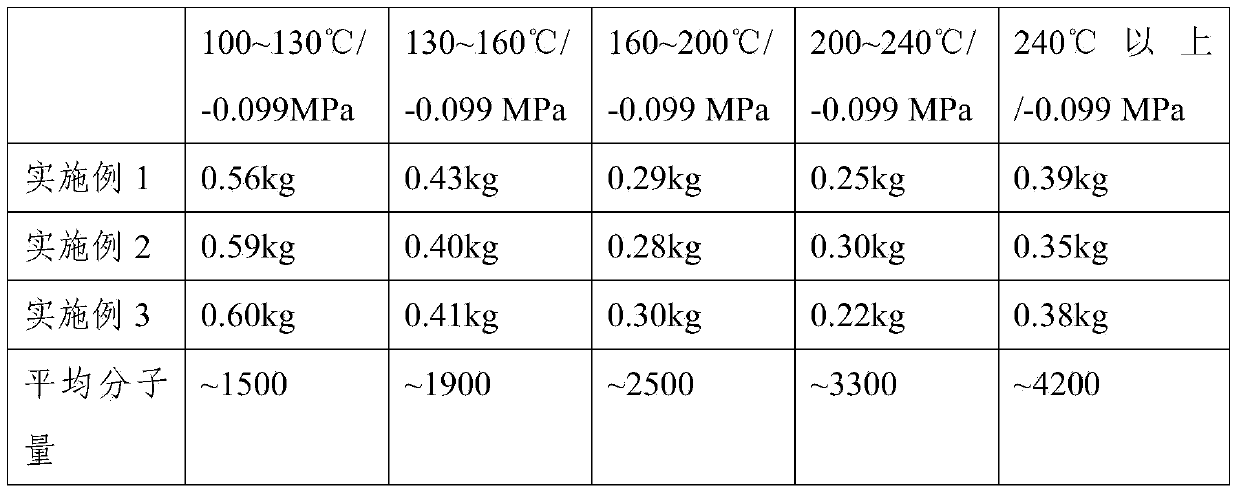
[0037] Self-made 5L stainless steel reaction kettle, built-in stirring, cooling coil, ultraviolet lamp, the measured peroxide value is 9.2×10 -2 Add 2kg of perfluoropolyether peroxide prepared by mmol / g photooxidative polymerization and 1.3kg of trifluorotrichloroethane into the reaction kettle, turn on the ultraviolet lamp (wavelength 365nm), when the temperature rises to 80°C, Slowly feed tetrafluoroethylene intermittently, the temperature is controlled at about 100°C to 105°C, the pressure is 1MPa, 30g of tetrafluoroethylene is added accumulatively, and 20g of tetrafluoroethylene is introduced per hour. . The peroxide value of the reacted product was measured to be 0.96×10 -6 mmol / g, the product is subjected to vacuum fractionation, and the fractions are shown in the table below.
Embodiment 3
[0039] Self-made 5L stainless steel reaction kettle, built-in stirring, cooling coil, ultraviolet lamp, the measured peroxide value is 9.2×10 -2 Add 2kg of perfluoropolyether peroxide prepared by mmol / g photooxidative polymerization into the reaction kettle, turn on the ultraviolet lamp (wavelength 365nm), and when the temperature rises to 80°C, intermittently and slowly pass tetrafluoroethylene into the kettle, The temperature is controlled at about 110°C to 120°C, the pressure is 1.2MPa, 30g of tetrafluoroethylene is added accumulatively, and 20g of tetrafluoroethylene is controlled to be introduced per hour. After passing through the tetrafluoroethylene, react for another 3 hours, and then cool down and discharge the material. The peroxide value of the reacted product was measured to be 0.53×10 -6 mmol / g, the product is subjected to vacuum fractionation, and the fractions are shown in Table 1 below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pov | aaaaa | aaaaa |
| Pov | aaaaa | aaaaa |
| Pov | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com