Process for removing newborn bovine serum endotoxin
A newborn bovine serum and endotoxin technology, applied to embryonic cells, vertebrate cells, animal cells, etc., can solve problems such as bacterial contamination, increase efficiency and added value, reduce endotoxin content, and lower serum protein concentration Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0009] Embodiment Removes newborn bovine serum endotoxin experiment
[0010] (1) Preparation of raw material newborn bovine serum
[0011] The newborn bovine serum with higher endotoxin content in the production process of the newborn bovine serum is used as the raw material bovine serum of the present invention.
[0012] The serum endotoxin content was detected using the Limulus Reagent Endotoxin Detection Kit from Zhanjiang Bokang Marine Biological Co., Ltd.
[0013] (2) Process for removing endotoxin from bovine serum
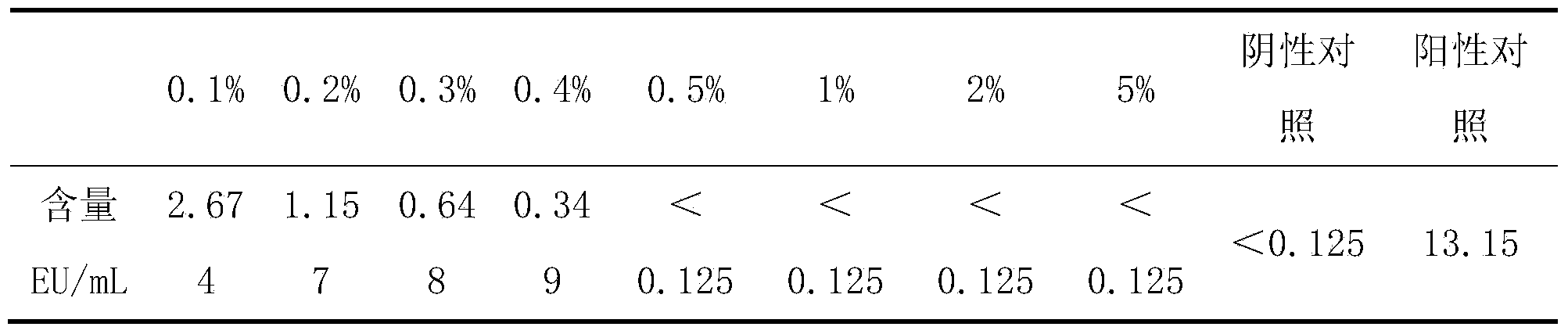
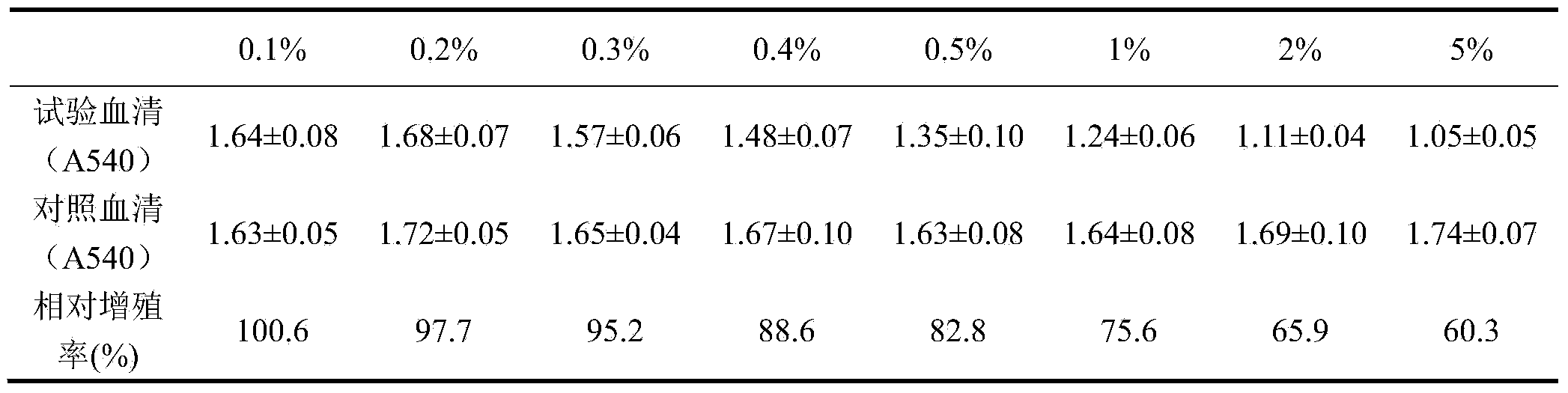
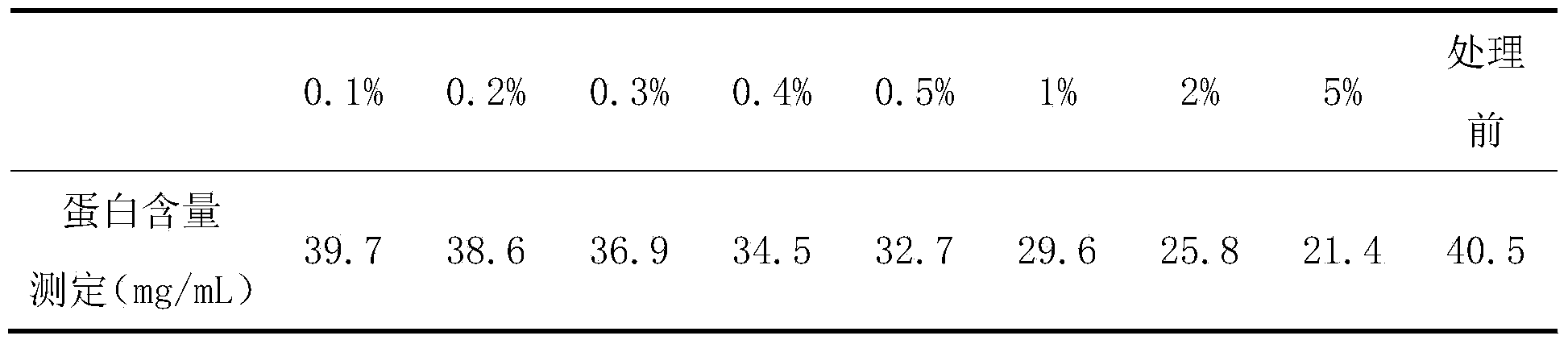
[0014] Slowly sprinkle the weighed different dosages of activated carbon (0.1%, 0.2%, 0.3%, 0.4%, 0.5%, 1%, 2%, 5%) into the serum and gently stir until uniform. After continuous gentle stirring for 30 minutes, Activated carbon was removed by filtration step by step to obtain newborn bovine serum. The filtered serum has been verified by the sterility test and meets the sterility requirements.
[0015] (3) Detection of endotoxin content
[0016] Dissolve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com