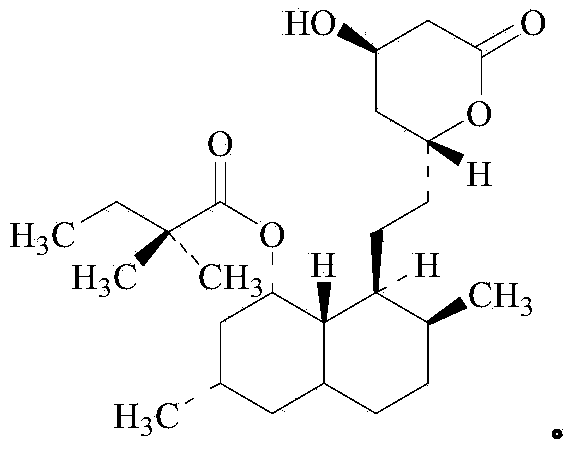
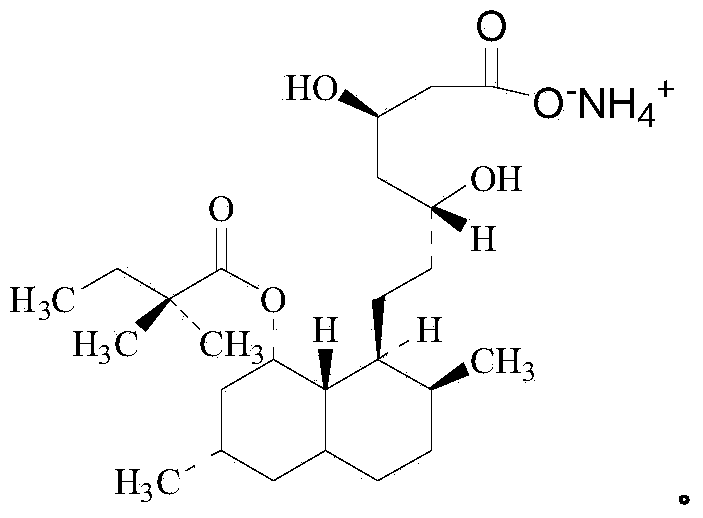
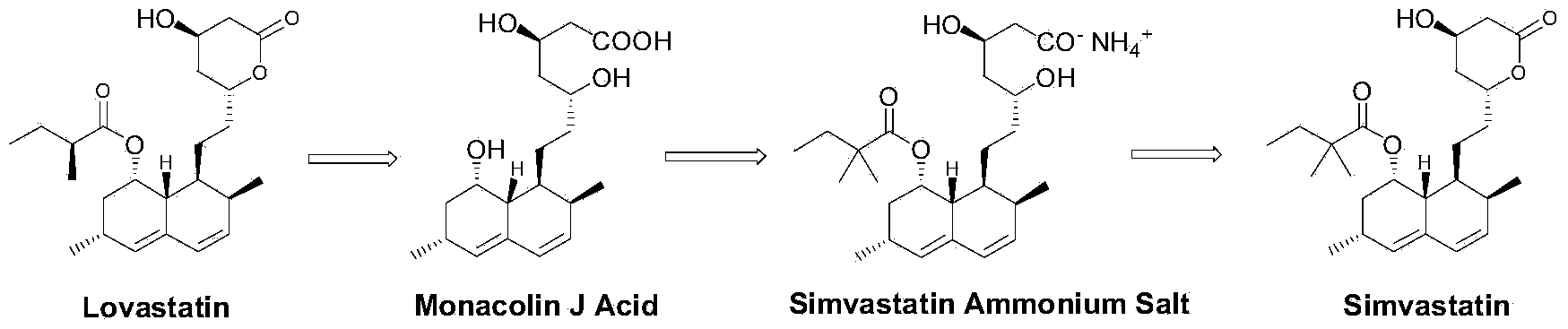
Preparation method of simvastatin ammonium salt
A simvastatin ammonium salt and substrate technology, applied in the field of medicine, can solve the problems of high production cost, large amount of solvent usage, poor stability, etc., and achieve the effects of increased production efficiency, reduced production cost, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] In a 50 mL three-necked flask, add 3 g of monacolin J acid (8.86 mmol, 1 eq), 30 mL of deionized water, and then add 1.81 g of ammonia (25% concentration by mass, 26.6 mmol, 3 eq). Adjust pH with 6M concentrated hydrochloric acid=8.6~8.9. Add 9.5mL recombinant acyltransferase enzyme solution, add 2,2-dimethylbutanoic acid-2-mercaptoethoxyacetate (2.9g, 13.3mmol, 1.5eq), 6M concentrated hydrochloric acid to adjust pH=8.6~ 8.9. The reaction process was monitored by HPLC, the reaction was 48 hours, and the conversion rate was 98.2%.
[0053] The reaction solution was centrifuged to obtain a solid. The solid was added to 20 mL of acetone for 4 hours to be slurried. The solid was dried under vacuum (40 degrees Celsius) to obtain a white crystalline product weighing 3.32 g, purity 97.9%, content 97.1%, yield 80.3 %.
Embodiment 2
[0055] In a 50 mL three-necked flask, add 3 g of monacolin J acid (8.86 mmol, 1 eq), 30 mL of deionized water, and then add 1.81 g of ammonia (25% concentration by mass, 26.6 mmol, 3 eq). 6M concentrated hydrochloric acid adjusts pH=8.6~8.9. Add activated carbon (100~200 mesh), add 3.17mL recombinant acyltransferase enzyme solution, add 2,2-dimethylbutyric acid-2-mercaptoethoxyacetate (2.9g, 13.3mmol, 1.5eq) , 6M concentrated hydrochloric acid adjust pH=8.6~8.9. The reaction process was monitored by HPLC, the reaction was 24 hours, and the conversion rate was 99.1%.
[0056] The reaction solution was centrifuged to obtain a solid. Add the solid to 15 mL of methanol, dissolve it at 40-50°C and then filter. The filtrate is concentrated to obtain a solid. After drying under vacuum (40°C), the white crystalline product weighs 3.45 g and has a purity of 98.5%. The content is 98.2%, and the yield is 82.0%.
Embodiment 3
[0058] In a 50 mL three-necked flask, add 3 g of monacolin J acid (8.86 mmol, 1 eq), 30 mL of deionized water, and then add 1.81 g of ammonia (25% concentration by mass, 26.6 mmol, 3 eq). Adjust pH with 6M concentrated hydrochloric acid=9.5~9.8. Add activated carbon (100~200 mesh), add 3.17mL recombinant acyltransferase enzyme solution, add 2,2-dimethylbutyric acid-2-mercaptoethoxyacetate (2.9g, 13.3mmol, 1.5eq) , 6M concentrated hydrochloric acid adjusts pH=9.5~9.8. The reaction process was monitored by HPLC. After 24 hours of reaction, the conversion rate was 99.3%.
[0059] The reaction solution was centrifuged to obtain a solid. The solid was added to 15 mL of methanol, dissolved at 40-50°C and then filtered. The filtrate was concentrated to obtain a solid, which was dried under vacuum (40°C) to obtain a white crystalline product weighing 3.63 grams with a purity of 99.5%. The content is 98.6%, and the yield is 89.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com