Method for producing lithium tetrafluoroborate solution
一种四氟硼酸锂、制造方法的技术,应用在四氟硼酸、最终产品制造、可持续制造/加工等方向,能够解决危险、水分残留、无法用锂电池用途等问题,达到反应收率高、反应的控制容易的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
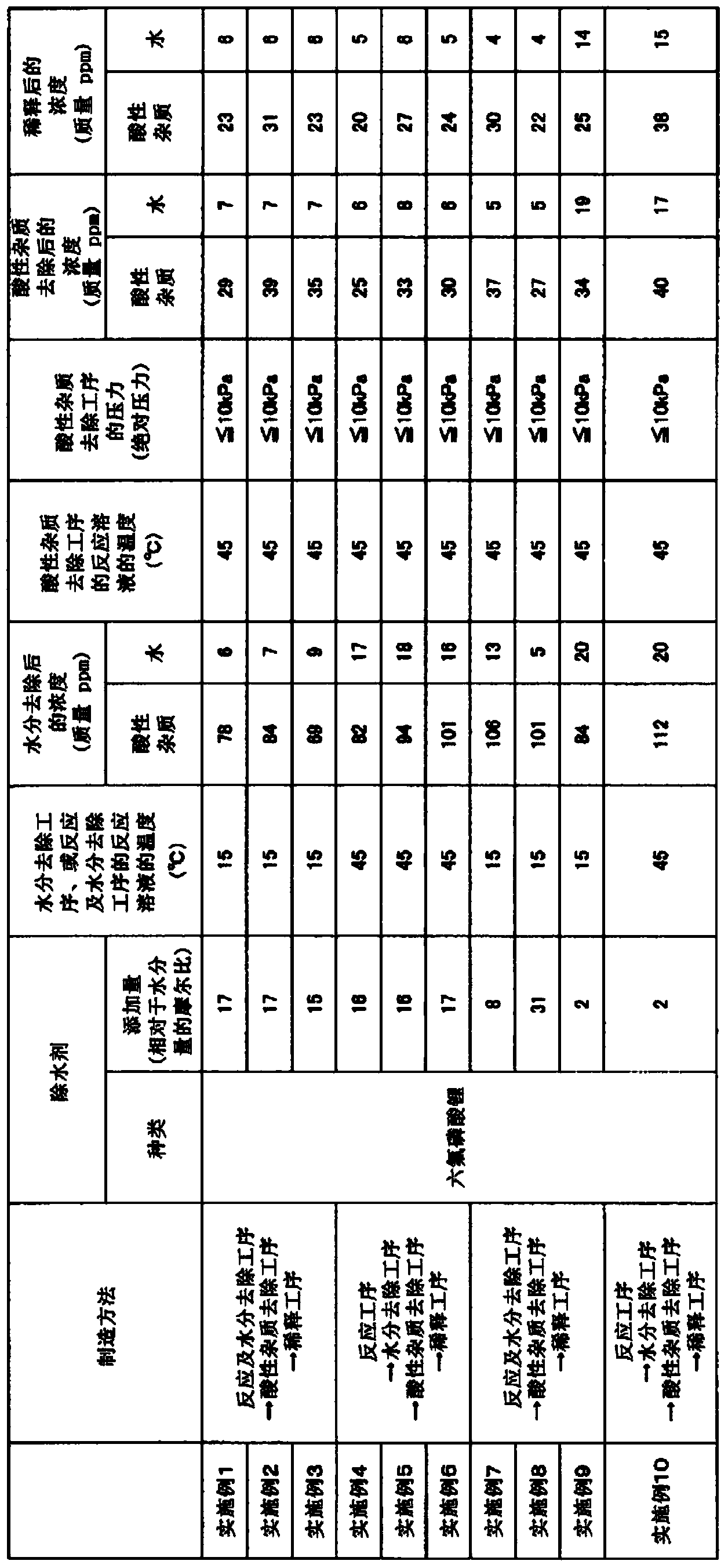
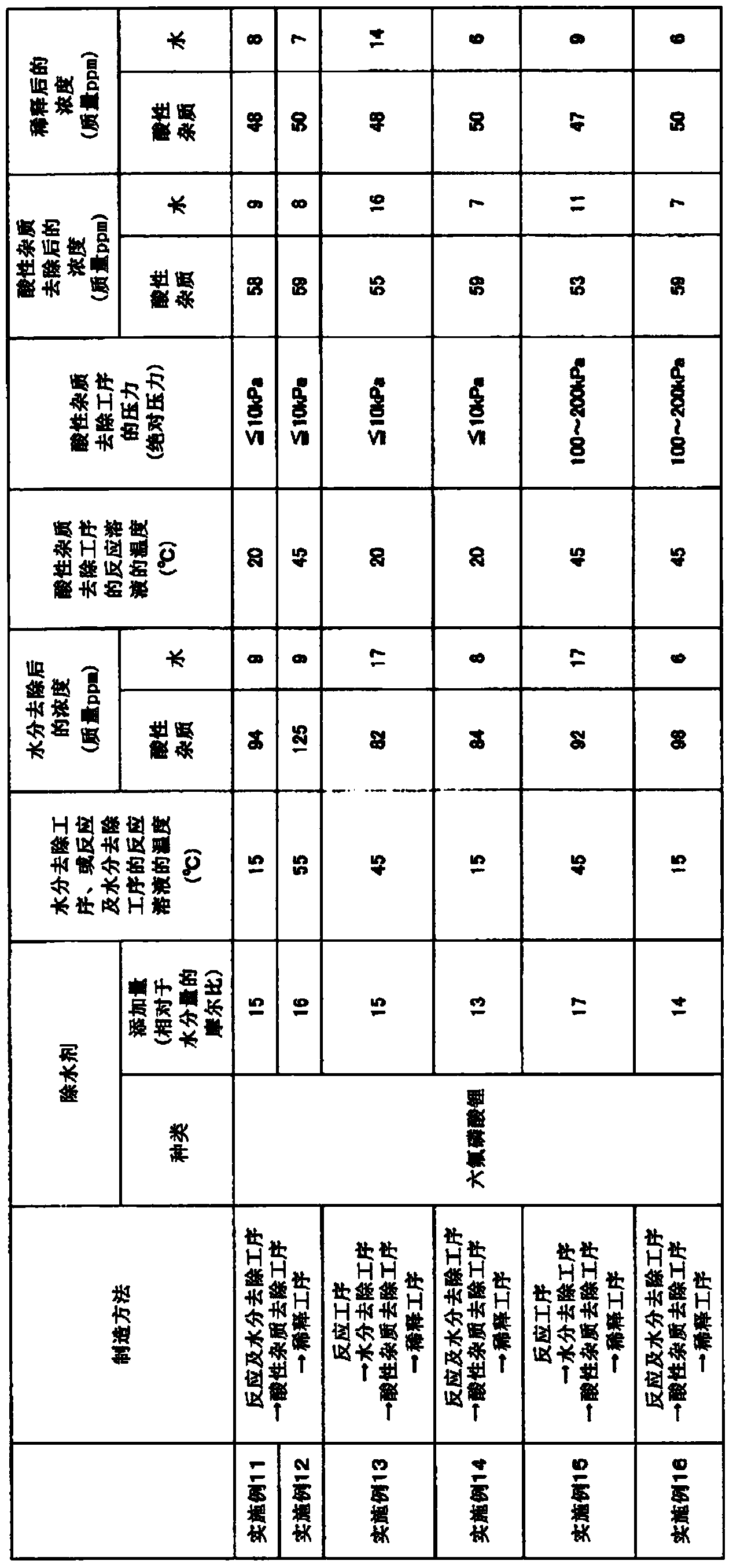
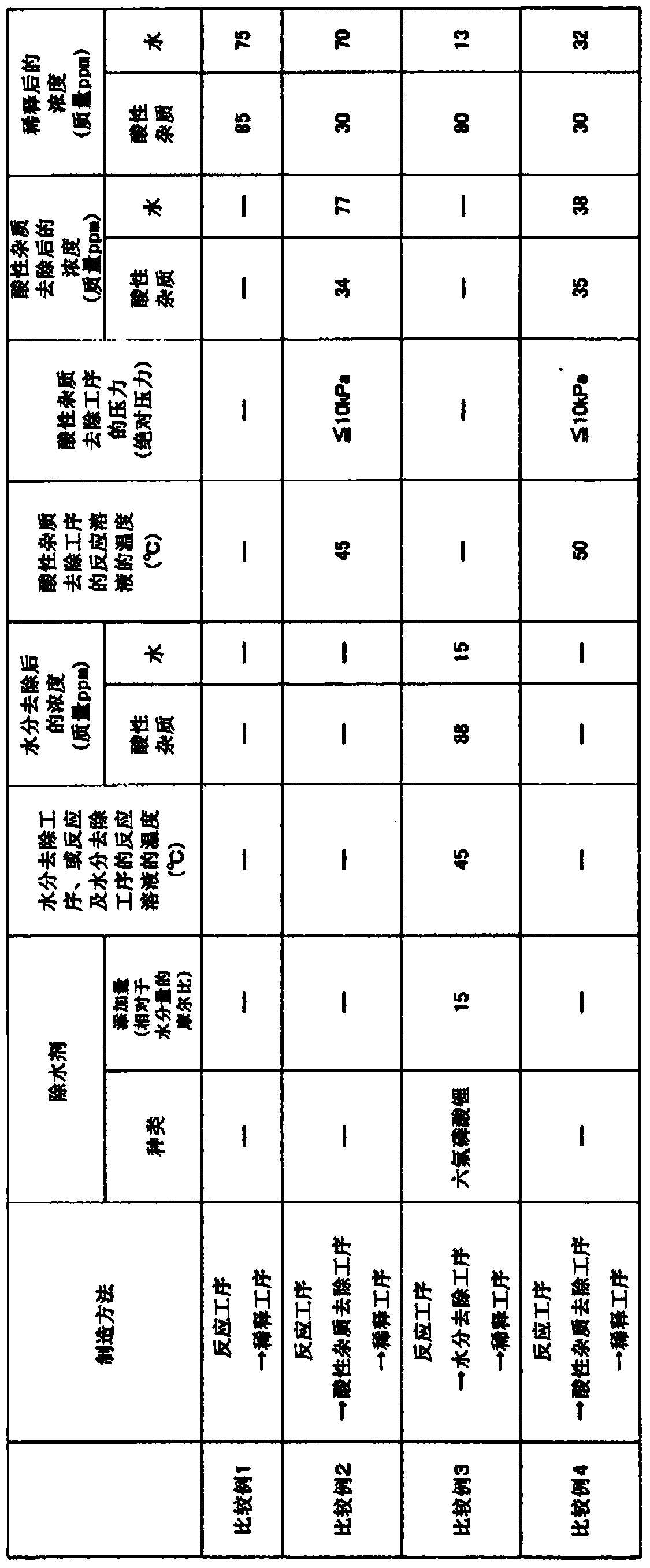
[0046] In a 500 mL three-necked glass flask, 18.8 g of lithium fluoride was added to 350.0 g of diethyl carbonate for mixing and dispersion. 4.1 g (27.0 mmol) of lithium hexafluorophosphate was added to the mixed dispersion liquid, cooled and maintained at 15°C, and boron trifluoride gas diluted with nitrogen to 62% by volume was passed through a gas introduction tube equipped with a mass flow controller. bubbling. The reaction was terminated when 44.7 g of boron trifluoride was consumed. Among them, the content of lithium hexafluorophosphate was 17 times the molar ratio of 1.56 mmol which is 0.028 g (moisture concentration: 68 mass ppm) of the total water contained in diethyl carbonate, lithium fluoride, and boron trifluoride. Lithium fluoride and boron trifluoride are reacted through the above steps to generate lithium tetrafluoroborate while removing water. The reaction solution obtained at this time had an acidic impurity concentration of 78 mass ppm, a water concentrati...
Embodiment 2
[0048] In a 500 mL three-necked glass flask, 18.8 g of lithium fluoride was added to 350.0 g of ethyl methyl carbonate, and mixed and dispersed. 4.1 g (27.0 mmol) of lithium hexafluorophosphate was added to the mixed dispersion liquid, cooled and maintained at 15°C, and boron trifluoride gas diluted with nitrogen to 63% by volume was passed through a gas introduction tube equipped with a mass flow controller. bubbling. The reaction was terminated when 44.8 g of boron trifluoride was consumed. Among them, the content of lithium hexafluorophosphate was 17 times the molar ratio of 1.61 mmol which is 0.029 g (moisture concentration: 70 mass ppm) of the total water contained in ethyl methyl carbonate, lithium fluoride, and boron trifluoride. Lithium fluoride and boron trifluoride are reacted through the above steps to generate lithium tetrafluoroborate while removing water. The reaction solution obtained at this time had an acidic impurity concentration of 84 mass ppm, a water co...
Embodiment 3
[0050] In a 500 mL three-necked glass flask, 18.8 g of lithium fluoride was added to 350.0 g of dimethyl carbonate for mixing and dispersion. 4.1 g (27.0 mmol) of lithium hexafluorophosphate was added to the mixed dispersion liquid, cooled and maintained at 15°C, and boron trifluoride gas diluted with nitrogen to 62% by volume was passed through a gas introduction tube equipped with a mass flow controller. bubbling. The reaction was terminated when 44.7 g of boron trifluoride was consumed. Among them, the content of lithium hexafluorophosphate was 15 times the molar ratio of 1.84 mmol which was 0.033 g (moisture concentration: 80 mass ppm) of the total water contained in dimethyl carbonate, lithium fluoride, and boron trifluoride. Lithium fluoride and boron trifluoride are reacted through the above steps to generate lithium tetrafluoroborate while removing water. The reaction solution obtained at this time had an acidic impurity concentration of 69 mass ppm, a water concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com