Microencapsulated preparation for protecting eyesight and preparation method thereof
A technology of microencapsulation and preparation, which is applied to medical preparations containing active ingredients, pharmaceutical formulations, active ingredients of hydroxyl compounds, etc., can solve the problems of low bioavailability and single formula, and achieve high bioavailability and uniform color. Long-lasting and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
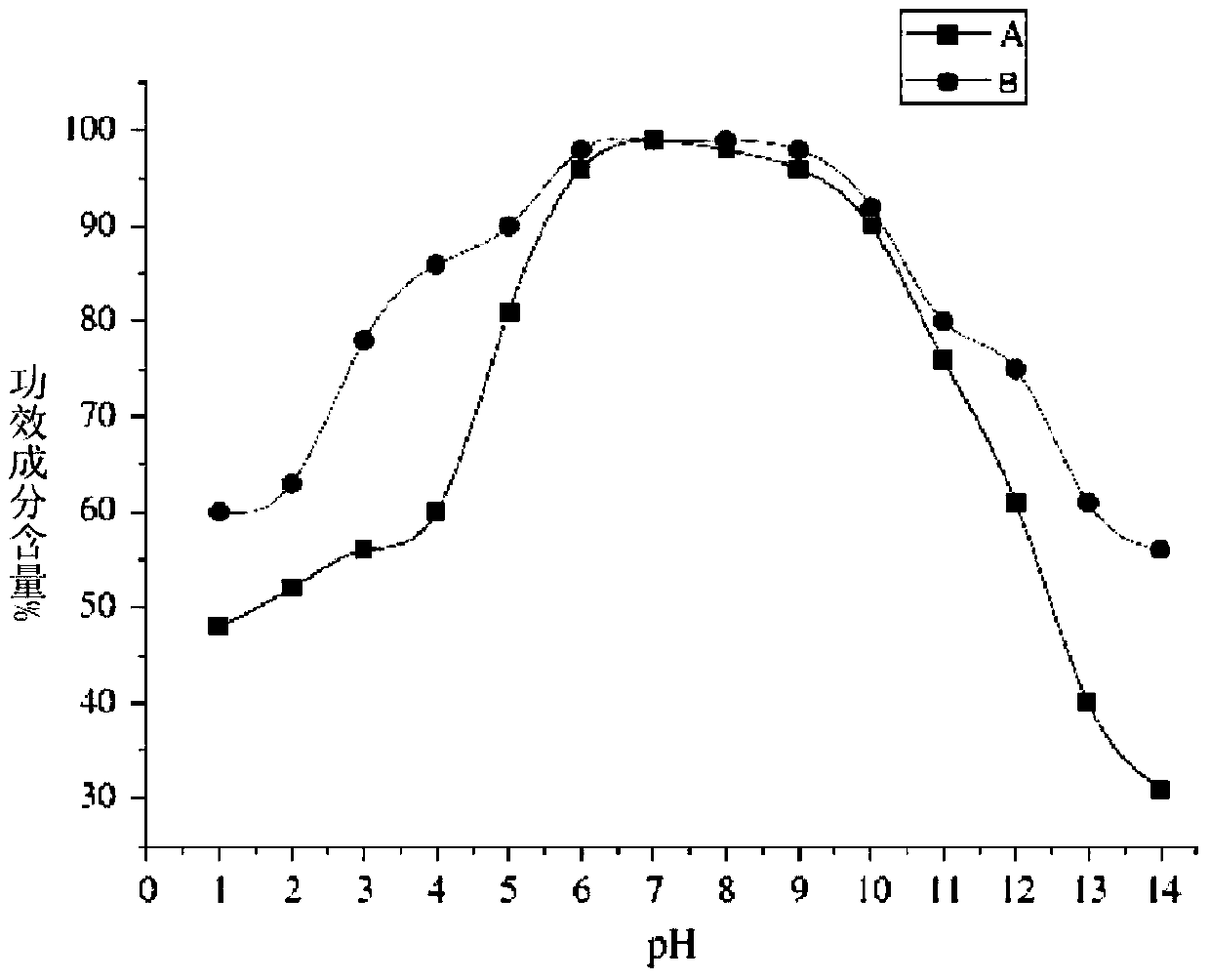
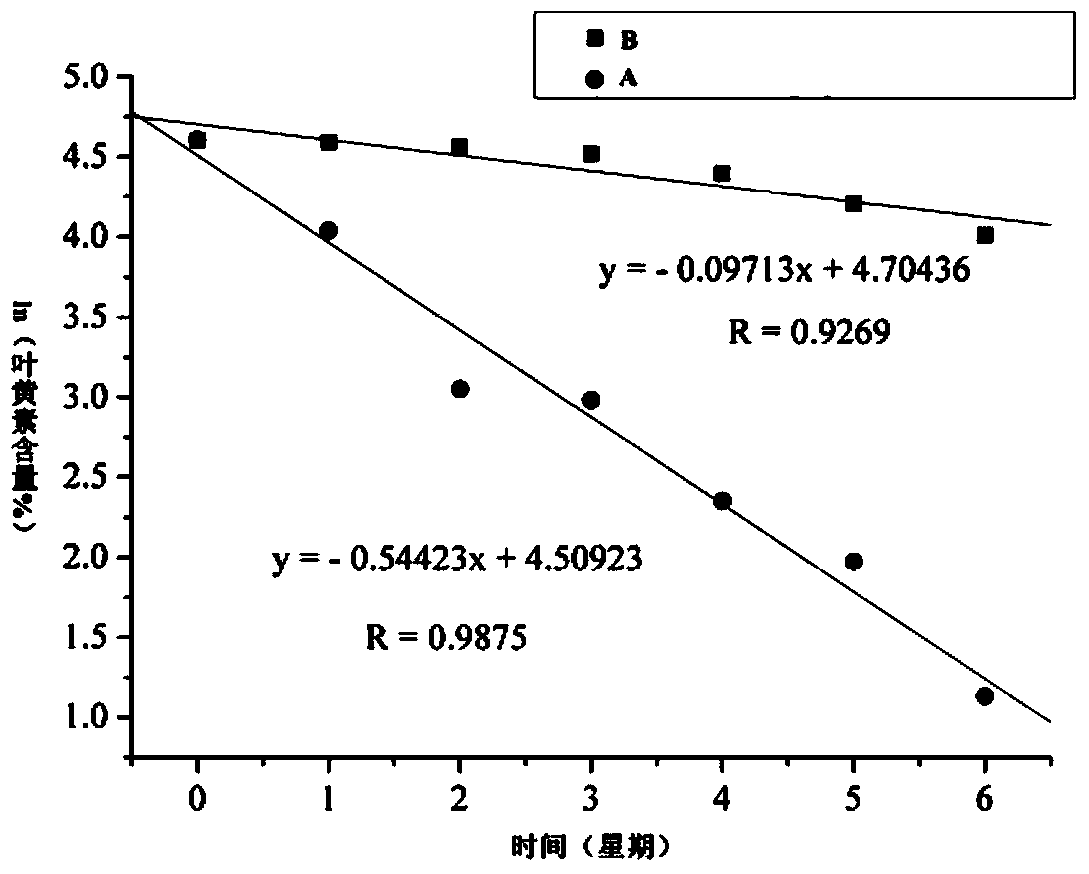
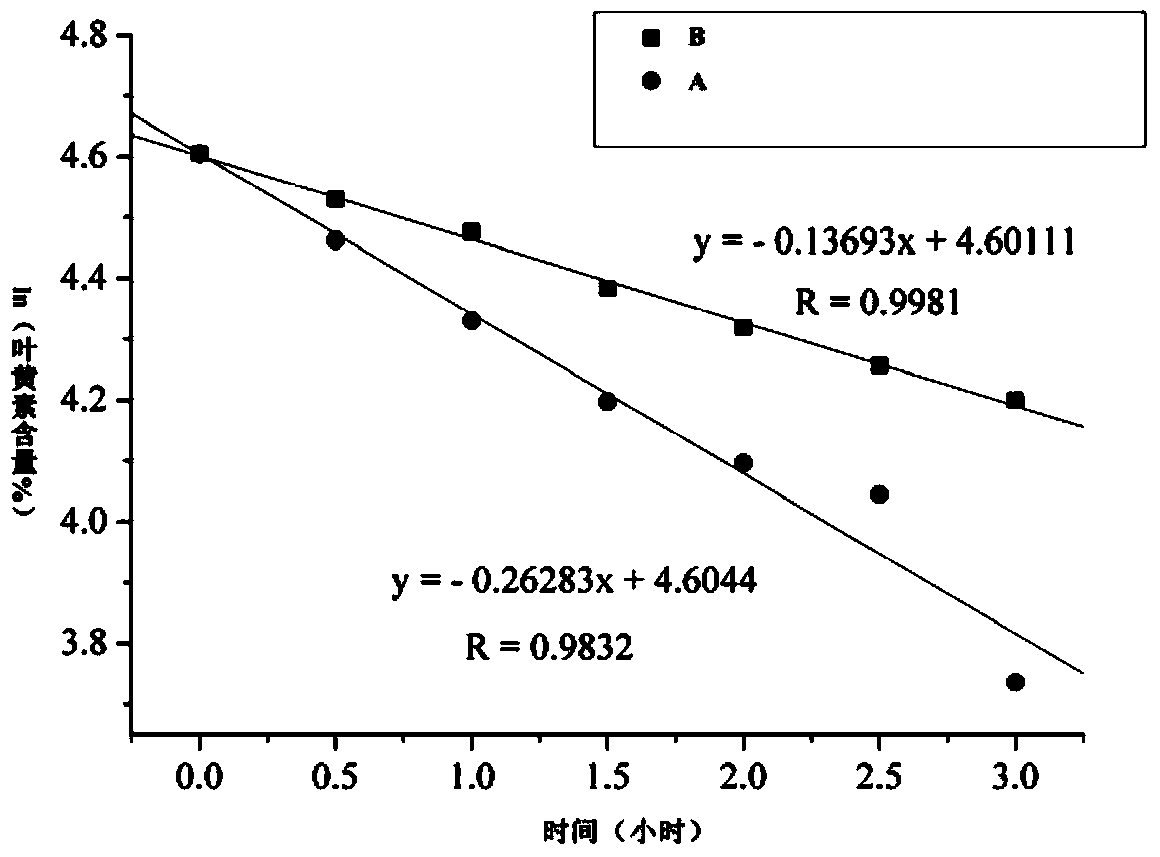
Image
Examples
Embodiment 1
[0037] Embodiment 1 compound lutein solid granule
[0038] formula:
[0039]
[0040] Preparation process: ①Add OSA modified starch, maltodextrin, sodium ascorbate and ascorbyl palmitate into 575ml of water at 50°C, stir and dissolve to obtain an aqueous phase solution; ②At 45°C, mix mixed tocopherols, sunflower Phospholipids and lutein crystals (purity 65%) were added to perilla seed oil, stirred and dissolved to obtain an oil phase solution; ③The water phase solution and the oil phase solution were mixed and emulsified for 5 hours, and the obtained emulsion was subjected to 20Mpa high pressure homogenization and spray drying to obtain cold water dispersion Type compound lutein-α-linolenic acid microcapsule powder, prepared into a compound lutein solid granule with a packaging specification of 4g / bag, and the dosage is 1 bag per day.
Embodiment 2
[0041] Example 2 Compound Lutein Ester Solid Granules
[0042] formula:
[0043]
[0044]
[0045] Preparation process: Same as in Example 1, the crystal purity of lutein ester is 80%, and the compound lutein ester solid granule is finally obtained, the packaging specification is 1g / bag, 1 bag per day.
Embodiment 3
[0046] Embodiment 3 Compound Lutein Ester Tablets
[0047] formula:
[0048]
[0049] Preparation process: The microencapsulation production process is the same as in Example 1, and the purity of lutein ester crystals is 80%. The prepared microcapsules are mixed with mannitol, vitamin C, calcium hydrogen phosphate, magnesium stearyl, polyethylene glycol, anhydrous citric acid and aspartame, and then granulated, dried, granulated, mixed and pressed into tablets The finished product is prepared, the specification is 1g / tablet, and the daily dosage is 4 tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com