Fusion protein of tnfα and dc-sign and its application
A technology of fusion protein and function, which is applied in the fusion protein of TNFα and DC-SIGN and its application field, which can solve the problems of single recognition site, affecting the overall function of monoclonal antibody, and poor binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The gene design of embodiment 1 fusion protein
[0062] 1. Linker peptide screening
[0063] The present invention first selects the linking peptide G10 as the linking peptide between TNFα and DC-SIGN.
[0064] Using SYFPEITHI, MH2pred, and ANNpred software, and using their default parameters for calculation, the T cell immunogenicity scores of G10 are calculated as: 5 (the highest score), 0, 0, and the B cell immunogenicity scores calculated by Bcepred and ABCpred are: 0, 0.51, the T cell and B cell immunogenicity scores calculated after normalization were 0.0 and 0.51, which belonged to weak immunogenicity.
[0065] 2. Protein model construction
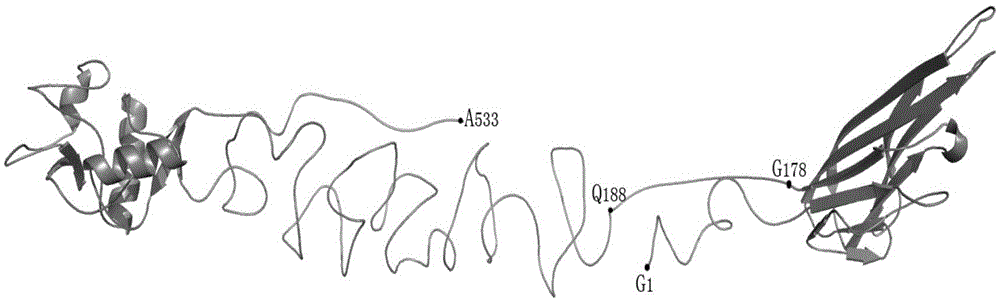
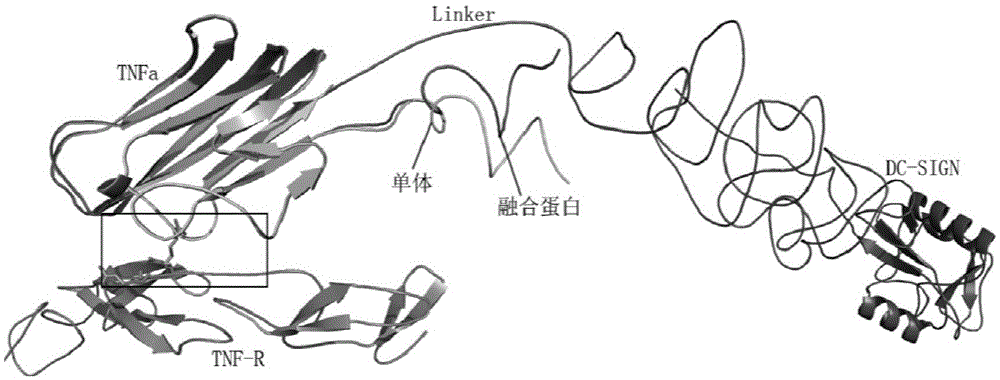
[0066] The present invention preferably adopts DC-SIGN as the recognition functional domain, and the GenBank number corresponding to DC-SIGN is M98457, and the corresponding protein number is AAF77072 ( figure 1), the full name of DC-SIGN is the specific intercellular adhesion factor 3 binding non-integrin molecule on the...
Embodiment 2A
[0075] The gene optimization of embodiment 2AT3002 fusion protein
[0076] 1. Codon optimization
[0077] In order to better express the AT3002 fusion protein, the applicant also carried out codon optimization, mRNA structure correction and translation initiation site optimization. The obtained gene sequence is shown in SEQ ID NO:1. Gene comparison before and after optimization such as figure 1 shown.
[0078] The following is a comparison of the parameters before and after the codon optimization of the AT3002 gene:
[0079] 1. Codon Adaptation Index (CodonAdaptationIndex, CAI)
[0080] Depend on Image 6 -a shows that before the codon optimization, the codon adaptation index (CAI) of the AT3002 gene in mammalian cells is 0.84. Depend on Image 6 -b It can be seen that after codon optimization, the CAI index of the AT3002 gene of the present invention in mammalian cells is 0.85. Usually, when CAI=1, it is considered that the gene is in the most ideal high-efficiency ex...
Embodiment 3A
[0089] Gene synthesis and expression vector construction of embodiment 3AT3002 fusion protein
[0090] The above-mentioned optimized AT3002 fusion protein gene was artificially synthesized, and HindIII and BamHI restriction sites were added to the two ends of the fusion protein gene. A stop codon site was also introduced behind the BamHI site to prevent the expression of the FLAG tag on the expression vector from affecting the properties of the fusion protein. The fusion protein gene was inserted into the p3xFLAG-CMV-13 plasmid ( Figure 9 a, The plasmid was purchased from Sigma), and a long-term storage plasmid was obtained, which was designated as p3xFLAG-CMV-13-AT3002 plasmid.
[0091] The whole gene of the above-mentioned optimized AT3002 fusion protein was artificially synthesized, and restriction sites of AvrⅡ and BstZ171 were added to the two ends of the full length of the fusion protein gene respectively. The fusion protein gene was inserted into the pCHO1.0 plasmid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com