Preparation method for lithium cobalt oxide anode material
A cathode material, lithium cobalt oxide technology, applied in chemical instruments and methods, battery electrodes, cobalt compounds, etc., can solve the problems of difficult commercial production, strong oxidizability and high valence state of the coating technology, and improve the cycle. Performance and safety performance, improved compaction density, uniform distribution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
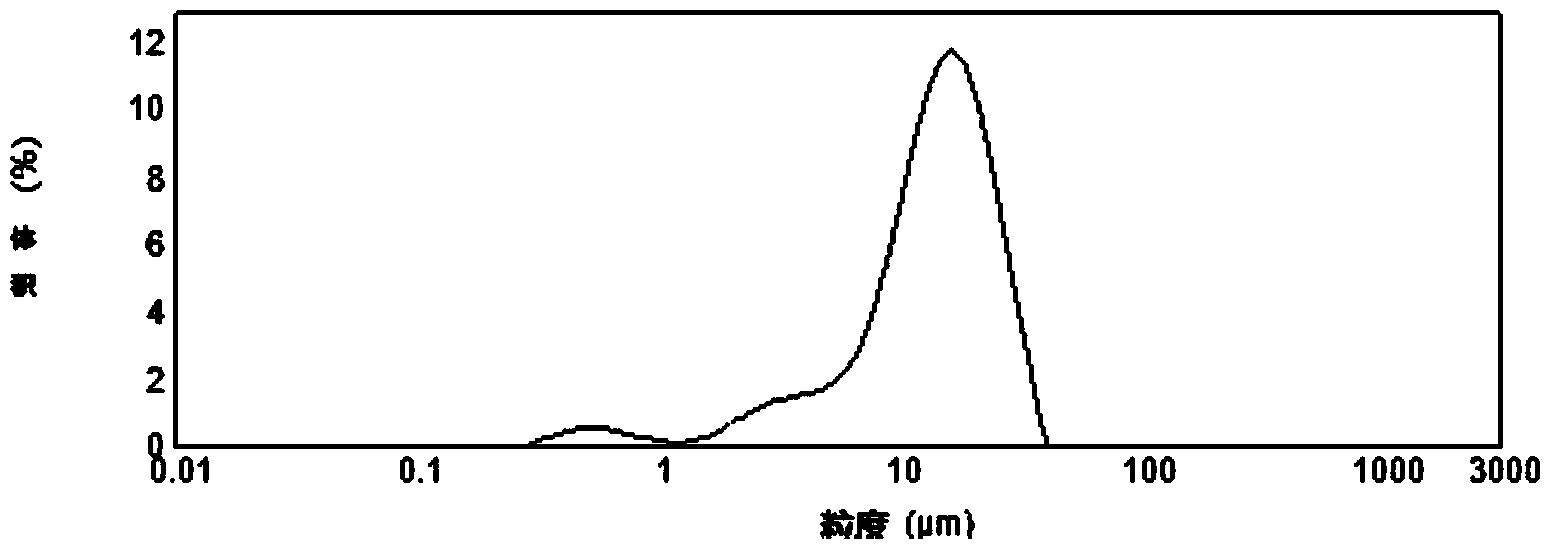
[0026] Dissolve CoCl2 in deionized water to make a salt solution with a Co concentration of 1.2mol / L, and prepare a NH4HCO3 solution with a concentration of 2mol / L. Add the two solutions into the reaction kettle slowly and dropwise, control the reaction temperature to 50°C, the stirring speed to 600r / min, and control the pH value in the early stage of the reaction to 7.5. The slurry overflows continuously during the precipitation process, and all the slurry returns to the reaction after suction filtration. The kettle continues to react, and the reaction time is about 15 days. The reaction product was washed and dried to obtain a spherical shape, D50: 15 μm, 0.45≤consistency≤0.55, and a cobalt carbonate precursor with obvious bimodal distribution can be seen in the particle size distribution diagram. The precursor was calcined at 700°C for 6 hours to obtain the raw material of tricobalt tetroxide.
[0027] According to Li / Co=1.03 (molar ratio), Mg addition amount is 2000ppm, w...
Embodiment 2
[0035] CoCl 2 Dissolve in deionized water to make a salt solution with a Co concentration of 1.2mol / L, and configure a NH solution with a concentration of 2mol / L 4 HCO 3 solution. Add the two solutions into the reaction kettle slowly and dropwise, control the reaction temperature to 50°C, the stirring speed to 600r / min, and control the pH value in the early stage of the reaction to 7.5. The slurry overflows continuously during the precipitation process, and all the slurry returns to the reaction after suction filtration. The kettle continued to react, and the reaction time was about 18 days. The reaction product was washed and dried to obtain a spherical shape, D50: 18 μm, 0.45≤consistency≤0.55, and the particle size distribution diagram showed an obvious bimodal distribution of the cobalt carbonate precursor. The precursor was calcined at 700°C for 6 hours to obtain the raw material of tricobalt tetroxide.
[0036] According to Li / Co=1.03 (molar ratio), the addition of Zr...
Embodiment 3
[0041] CoCl 2 Dissolve in deionized water to make a salt solution with a Co concentration of 1.2mol / L, and configure a NH solution with a concentration of 2mol / L 4 HCO 3 solution. Add the two solutions into the reaction kettle slowly and dropwise, control the reaction temperature to 50°C, the stirring speed to 600r / min, and control the pH value in the early stage of the reaction to 7.5. The slurry overflows continuously during the precipitation process, and all the slurry returns to the reaction after suction filtration. The kettle continues to react, and the reaction time is about 15 days. The reaction product was washed and dried to obtain a spherical shape, D50: 15 μm, 0.45≦consistency≦0.55, and a cobalt carbonate precursor with obvious bimodal distribution characteristics can be seen from the particle size distribution diagram. The precursor was calcined at 700°C for 6 hours to obtain the raw material of tricobalt tetroxide.
[0042] According to Li / Co=1.03 (molar rati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com