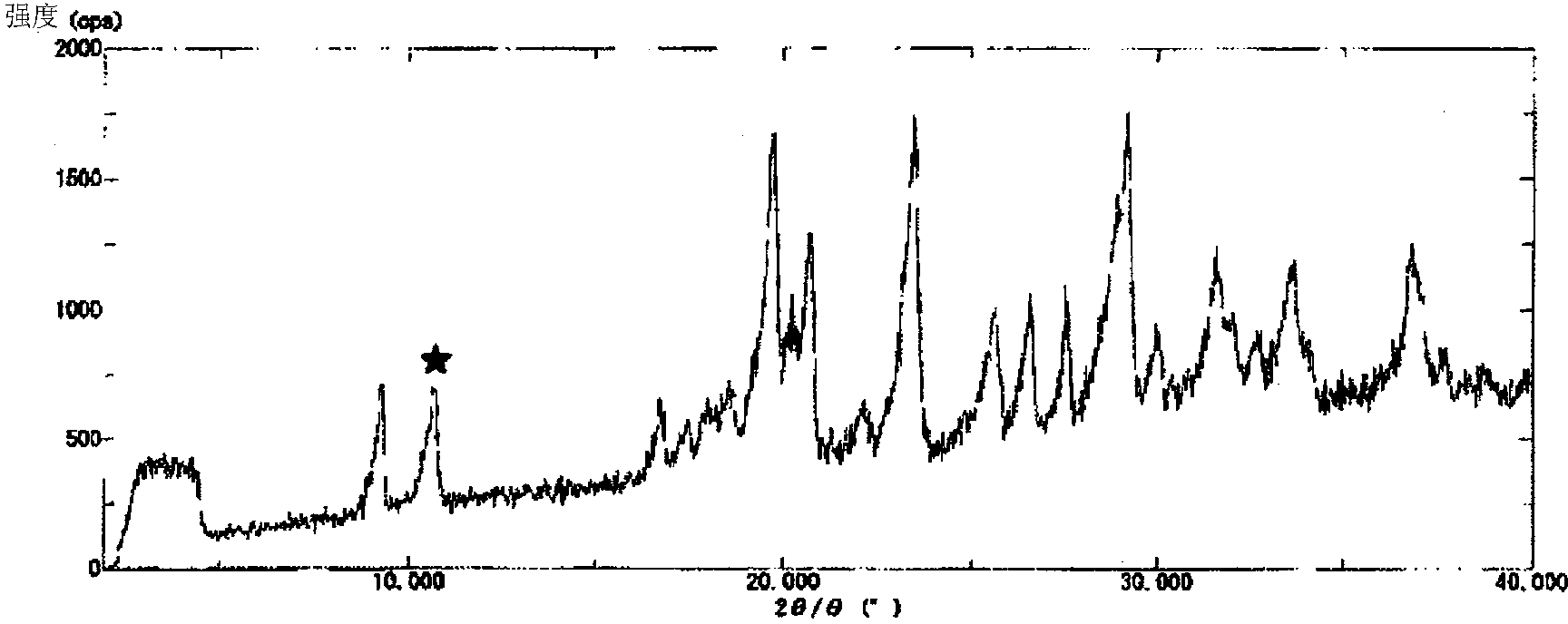
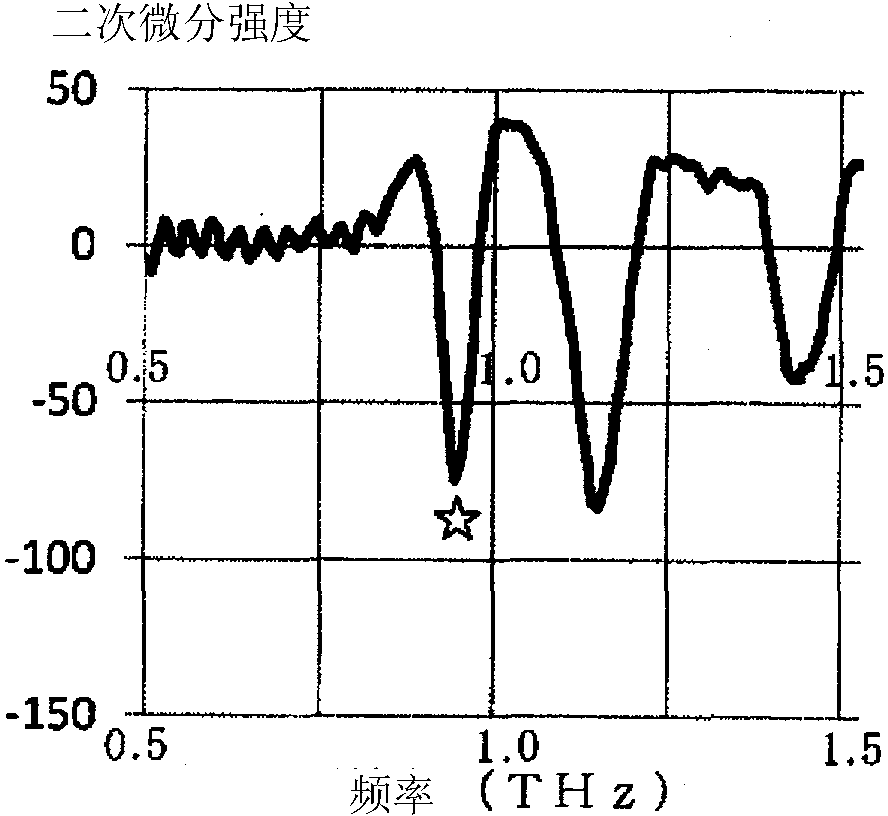
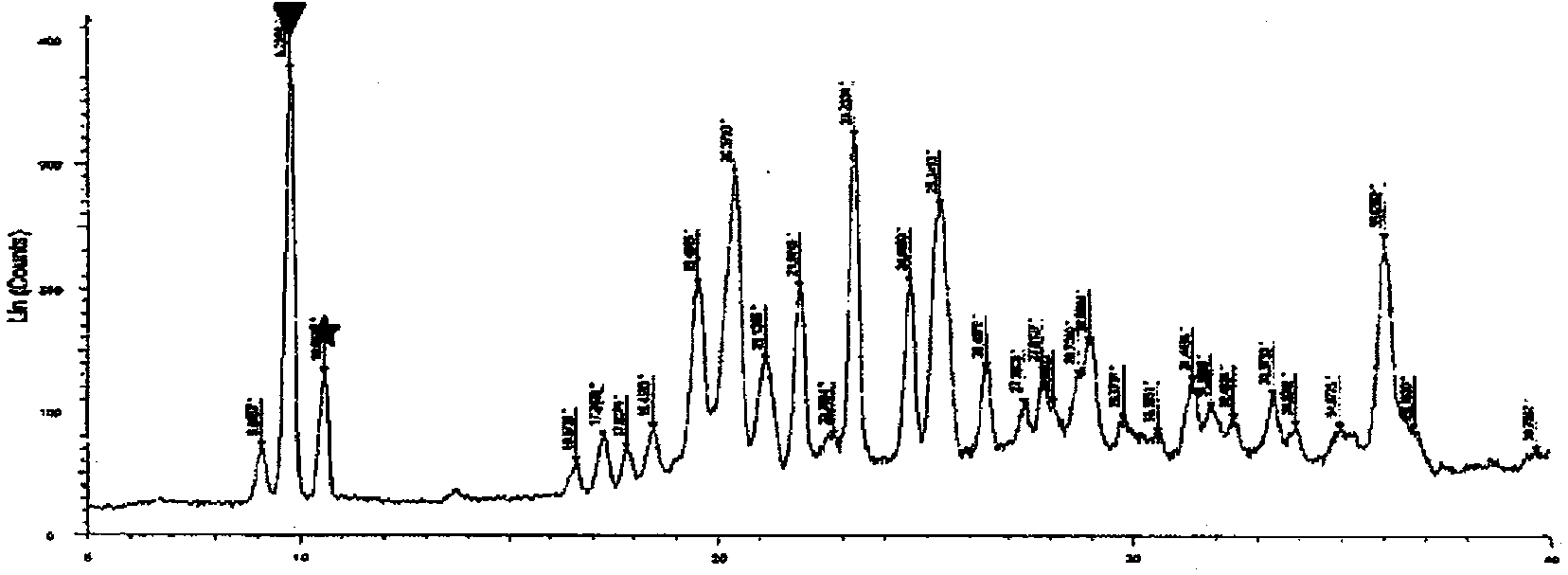
Nicorandil-containing pharmaceutical composition
A technology of nicorandil and composition, applied in the field of pharmaceutical composition containing nicorandil, can solve problems such as less than 1 year, and achieve the effect of reducing risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0075] Next, although an Example is shown and this invention is demonstrated more concretely, this invention is not limited to this.
preparation example 1
[0077] Weigh 300 mg of Nicorandil in the Japanese Pharmacopoeia, 75 mg of D-mannitol (trade name: Mannit S, Mitsubishi Corporation Furd Teck Co., Ltd.) and 300 mg of sodium citrate hydrate (Wako Pure Chemical Industries, Ltd.), and dissolve in To distilled water for injection, citric acid (Wako Pure Chemical Industries, Ltd.) and / or sodium hydroxide (Wako Pure Chemical Industries, Ltd.) were added as necessary to adjust the pH to a range of 7.0 to 8.5 to make the total volume 50 mL. After filtering this solution with a 0.22 μm membrane filter, 8 mL per bottle was dispensed into glass vials with a bottle diameter of 33.0 mm, and a freeze-drying device (trade name: TriomasterII-A04, Kyowa Vacuum Technology Co., Ltd.) was used to dry at -40 ℃ for pre-freezing and freeze-drying. In the initial cooling step in the freeze-drying step, heat treatment (annealing) was performed twice for 6 hours at a product temperature (product temperature) of 0°C. During the heat treatment step, the...
preparation example 2、6、7、11、13、14
[0079] According to the production method of formulation example 1, a freeze-dried formulation was obtained with the formulation shown in Table 1 (content of each component per glass vial).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com