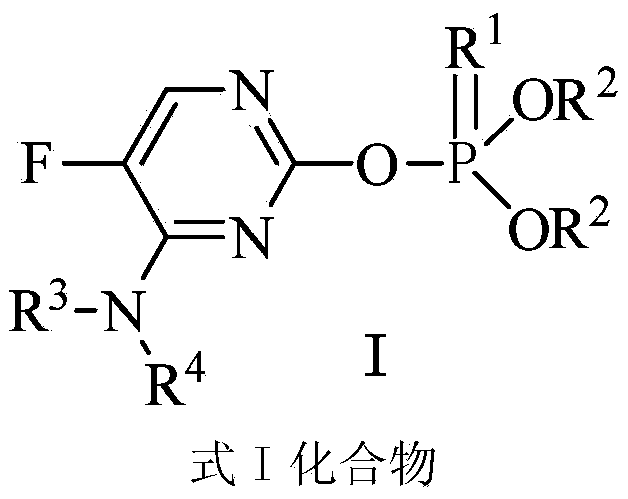
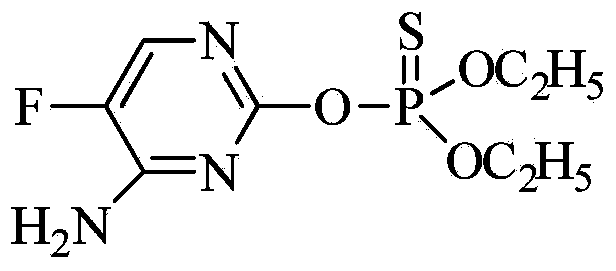
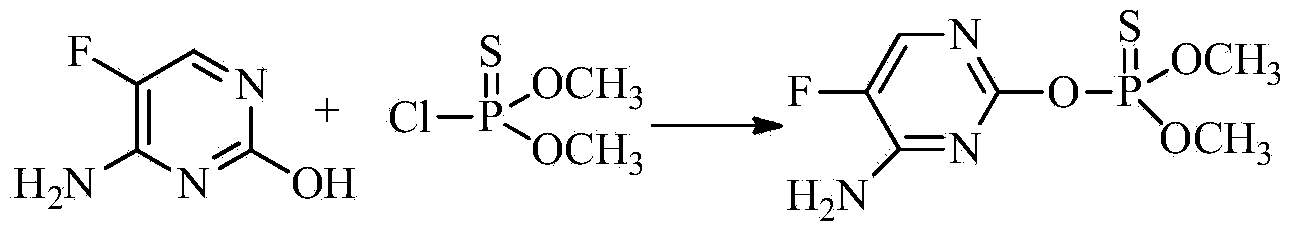Pyrimidine-containing organophosphorus fungicide and application
A fungicide, organophosphorus technology, applied in the directions of fungicides, applications, biocides, etc., can solve problems such as the inability to obtain compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Preparation of O-(4-amino-5-fluoropyrimidin-2-yl)O,O-dimethylphosphorothioate
[0130]
[0131]In a 1000 ml four-necked flask with a stirring and thermometer, add 500 ml of anhydrous N,N-dimethylformamide, add 0.1 mole of 5-fluorocytosine, and slowly add 0.1 mole of metal After adding sodium, stir for 2 hours, add O, O-dimethylphosphoryl thiochloride, stir at room temperature for 48 hours, distill N, N-dimethylformamide at 1 mmHg until the solvent is distilled clean, and the distillation temperature is not When the temperature is greater than 80 degrees, the obtained product is dissolved in 500 ml of methanol, filtered to remove the salt, and the methanol is distilled off to obtain the crude product of O-(4-amino-5-fluoropyrimidin-2-yl)O,O-dimethyl phosphorothioate , the content was 78% by liquid chromatography analysis, and the yield was 80%. Using ethyl acetate-n-hexane (4:1) as the chromatographic solution, the pure product was obtained. The calculated data of e...
Embodiment 2
[0140] O-(4-acetamido-5-fluoropyrimidin-2-yl)OO-diethyl phosphorothioate
[0141]
[0142] In a 1000 ml four-neck flask with stirring and a thermometer, add 500 ml of anhydrous N,N-dimethylformamide, add 0.1 mole of 5-fluoroacetylcytosine, and slowly add 0.1 mole of Metal sodium, after adding, stir for 2 hours, add O, O-dimethylphosphoryl thiochloride, stir at room temperature for 48 hours, distill N,N-dimethylformamide at 1mmHg until the solvent is distilled clean, the distillation temperature Not more than 80 degrees, the obtained product was dissolved in 500 ml of methanol, filtered to remove the salt, and the methanol was distilled off to obtain O-(4-acetamido-5-fluoropyrimidin-2-yl)O,O-diethylthio The crude phosphoric acid ester was analyzed by liquid chromatography with a content of 42% and a yield of 39%. Purified by column chromatography using ethyl acetate-n-hexane (6:4) as the chromatographic liquid, the pure product was obtained, and the calculated data of eleme...
Embodiment 3
[0144] O-(5-fluoro-4-(methylamino)pyrimidin-2-yl)O,O-dimethylphosphorothioate
[0145]
[0146] In a 100 ml four-neck flask with stirring and a thermometer, add 50 ml of anhydrous methyl ethyl ketone, add 0.01 mole of 5-fluoro 4-methylamino-pyrimidin-2-one, and slowly add 0.011 mole of After adding sodium hydroxide, stir for 2 hours, add O, O-dimethylphosphoryl thiochloride, stir at room temperature for 96 hours, distill butanone at 10 mmHg until the solvent is distilled clean, the distillation temperature is not more than 40 degrees, and the residual After dissolving with 500 ml of toluene at night, remove the salt by filtration, and distill off the toluene to obtain the crude product of O-(5-fluoro-4-(methylamino)pyrimidin-2-yl)O,O-dimethyl phosphorothioate. The liquid chromatographic analysis showed that the content was 56%, and the yield was 49%. Purified by column chromatography using ethyl acetate-n-hexane (6:4) as the chromatographic fluid, the pure product was obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com