Catalyst for surface sulfation of ferric oxide, as well as preparation method and application thereof
A technology of iron oxide and sulfation, which is applied in the directions of physical/chemical process catalysts, chemical instruments and methods, separation methods, etc., can solve the problem that the application of medium temperature and low temperature cannot be significantly improved, the specific process of iron oxide material is not disclosed, and it is unfavorable for industrialization. application and other issues, to achieve the effects of low price, environmental friendliness, and improved reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A preparation method of a surface sulfated iron oxide catalyst, said method comprising the steps of:
[0058] (1) Using ferric nitrate as Fe source and ammonia water as precipitant, Fe was prepared by precipitation method 2 o 3 : Prepare a certain concentration of Fe(NO 3 ) 3 Solution, with the ammoniacal liquor of 25wt% as precipitating agent, use alkaline burette to add ammoniacal liquor dropwise in mixed solution, rate of addition is controlled at about 1 drop / second, adjust solution pH value to 9, make Fe ion precipitation completely; Precipitate is carried out Suction filtration and washing, and then place the suction filtered filter cake in an oven and dry at 100°C for 12 hours; place the dried sample in a muffle furnace, control the heating rate at 5°C / min, and place it in an air atmosphere at 500°C Roasting 3h. After roasting and cooling Fe 2 o 3 The samples were ground and sieved, and 20-60 mesh particles were selected for use.
[0059] (2) Using SO 2 I...
Embodiment 2
[0061] The same method as in Example 1 was adopted, except that the processing time of step (2) was 8 hours.
Embodiment 3
[0063] The same method as in Example 1 was adopted, except that the processing time of step (2) was 24 hours.
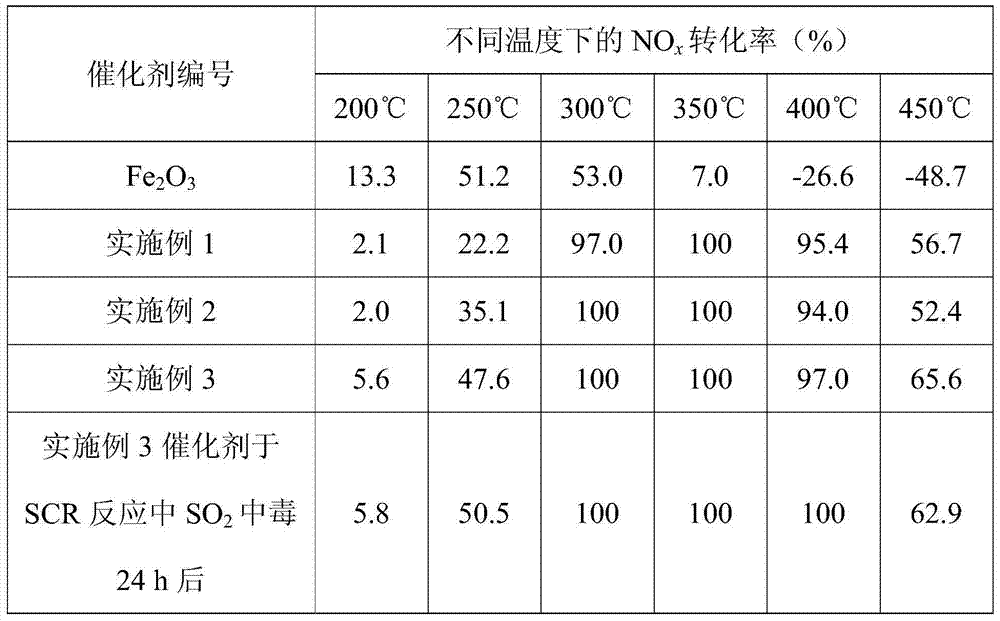
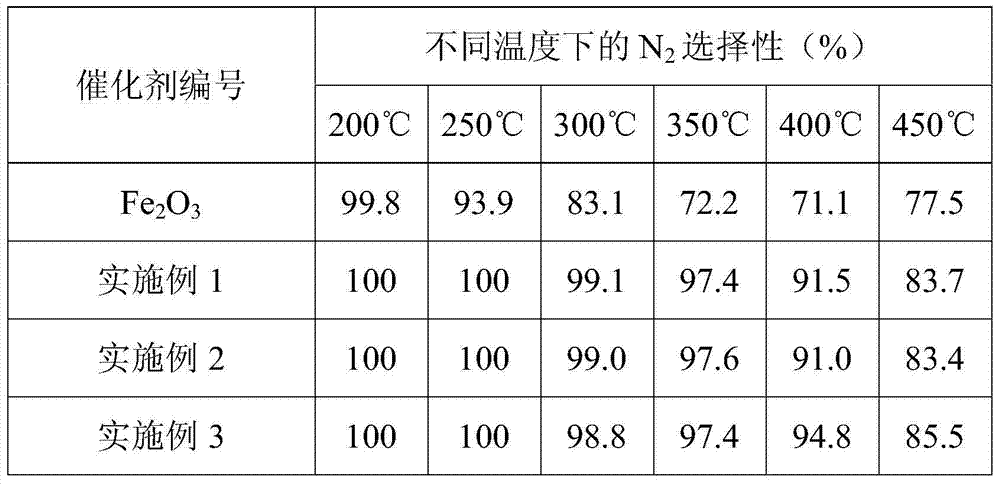
[0064] Take pure Fe 2 o 3 Material and embodiment 1~3 different SO 2 The surface sulfated iron oxide catalyst obtained during the treatment time, with a catalyst volume of 0.6mL and 40-60 meshes, was put into a catalyst activity evaluation device, and the activity evaluation was carried out in a fixed-bed reactor. The simulated flue gas composition is (500ppm NH 3 , 500ppm NO, 5%O 2 ), N 2 It is the balance gas, the total flow rate is 500mL / min, and the reaction space velocity is 50000h -1 . The test results are shown in Table 1 and Table 2.
[0065] Table 1 Different catalysts in NH 3 -NO in SCR reaction x Conversion rates
[0066]
[0067] Table 2 Different catalysts in NH 3 -N in SCR reaction 2 selectivity
[0068]
[0069] Pure Fe 2 o 3 MaterialNH 3 - SCR catalytic activity is very low, highest NO x The conversion rate is only about 50%, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com