Tetrahydroisoquinoline derivative and synthesis method thereof
A technology of tetrahydroisoquinoline and its derivatives, applied in organic chemistry and other fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
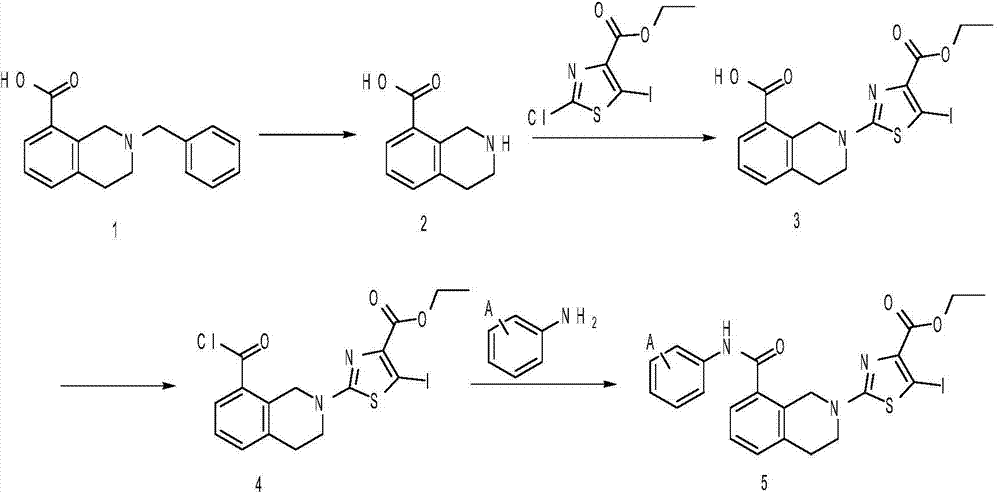
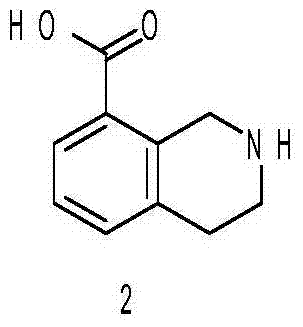
[0026] (1) Synthesis of 1,2,3,4-tetrahydroisoquinoline-8-carboxylic acid
[0027] Add 100 g of 2-benzyl-1,2,3,4-tetrahydroisoquinoline-8-carboxylic acid into 2L of methanol, then add 10 g of 10% Pd / C, pass in hydrogen, stir the reaction at room temperature for 24 hours, stop React, filter, and concentrate the filtrate under reduced pressure to obtain 1,2,3,4-tetrahydroisoquinoline-8-carboxylic acid.
[0028] (2) Synthesis of 2-(4-(ethoxycarbonyl)-5-iodothiazol-yl)-1,2,3,4-tetrahydroisoquinoline-8-carboxylic acid
[0029] Add 60g of 1,2,3,4-tetrahydroisoquinoline-8-carboxylic acid, 90g of ethyl 2-chloro-5-iodothiazole-4-carboxylate, and 55g of anhydrous potassium carbonate to 800ml N,N- In dimethylformamide, heat to reflux, stir for 10 hours, stop the reaction, remove most of N,N-dimethylformamide under reduced pressure, extract the residue with ethyl acetate and water, and collect the organic phase. After drying, concentration and separation on a silica gel column, 136.8 g o...
Embodiment 2
[0036] Synthesis of ethyl-5-iodo-2-(8-(p-methylphenylcarbamoyl)-3,4-dihydroisoquinolin-2(1H)-yl)thiazole-4-carboxylic acid ethyl ester
[0037] Add 35g of p-methylaniline to 800ml of ethyl acetate, then add 50ml of pyridine, cool down to 0°C, and dropwise add 125g of ethyl 2-(8-(chlorocarbonyl)-3,4-dihydroisoquinoline-2 (1H)-yl)-5-iodothiazole-4-carboxylic acid ethyl ester, keep the temperature of the system not exceeding 10°C, after the dropwise addition, continue to stir at 0°C for 14 hours, slowly pour the reaction solution into ice water, add dilute hydrochloric acid, stirred, separated, collected the organic phase, dried, concentrated and separated on a silica gel column to obtain 129.8g of ethyl-5-iodo-2-(8-(p-methylphenylcarbamoyl)-3,4-di Hydroisoquinolin-2(1H)-yl)thiazole-4-carboxylic acid ethyl ester.
[0038] H1-NMR (CDCl 3 ,400M):8.72(1H,brs),7.76~7.65(3H,m),7.34~7.26(4H,m),7.17(1H,m),4.26~4.12(4H,m),3.42(2H,t ), 2.66(2H,t), 2.31(3H,s), 1.31(3H,t).
Embodiment 3
[0040] Ethyl-5-iodo-2-(8-(m-methoxyphenylcarbamoyl)-3,4-dihydroisoquinolin-2(1H)-yl)thiazole-4-carboxylic acid ethyl ester synthesis
[0041]Add 40g of m-methoxyaniline to 800ml of ethyl acetate, then add 50ml of pyridine, cool down to 0°C, and dropwise add 125g of ethyl 2-(8-(chlorocarbonyl)-3,4-dihydroisoquinoline- 2(1H)-yl)-5-iodothiazole-4-carboxylic acid ethyl ester, keep the temperature of the system not exceeding 10°C, after the dropwise addition, continue to stir at 0°C for 14 hours, slowly pour the reaction solution into ice water, add dilute hydrochloric acid, stirred, separated, collected the organic phase, dried, concentrated and separated on a silica gel column to obtain 132.3g of ethyl-5-iodo-2-(8-(p-methylphenylcarbamoyl)-3,4- Ethyl dihydroisoquinolin-2(1H)-yl)thiazole-4-carboxylate.
[0042] H1-NMR (CDCl 3 ,400M):8.73(1H,brs),7.79~7.67(5H,m),7.35~7.26(2H,m),7.15(1H,m),4.23~4.11(4H,m),3.78(3H,s ), 3.46(2H,t), 2.67(2H,t), 1.32(3H,t).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com