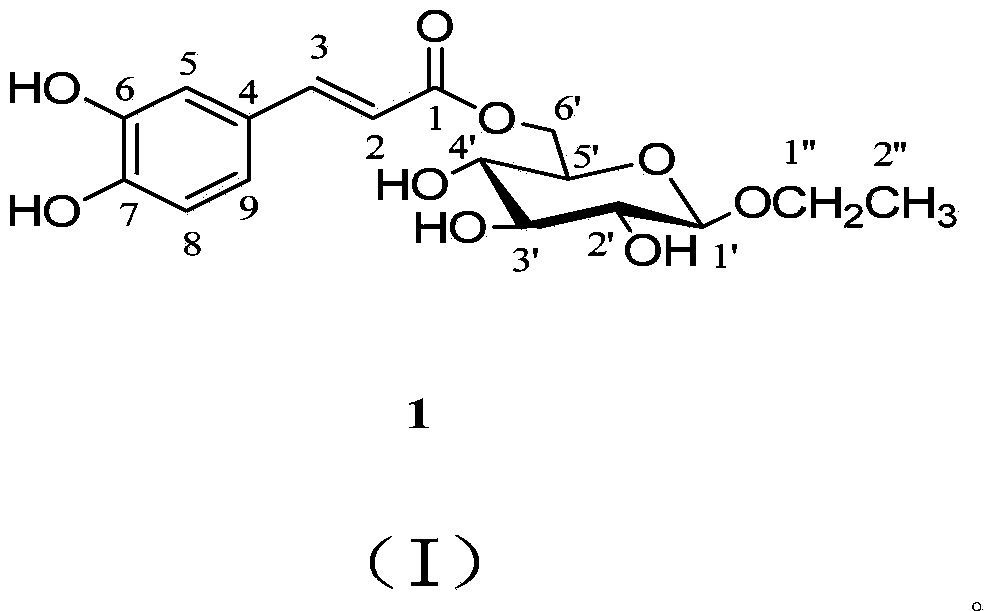
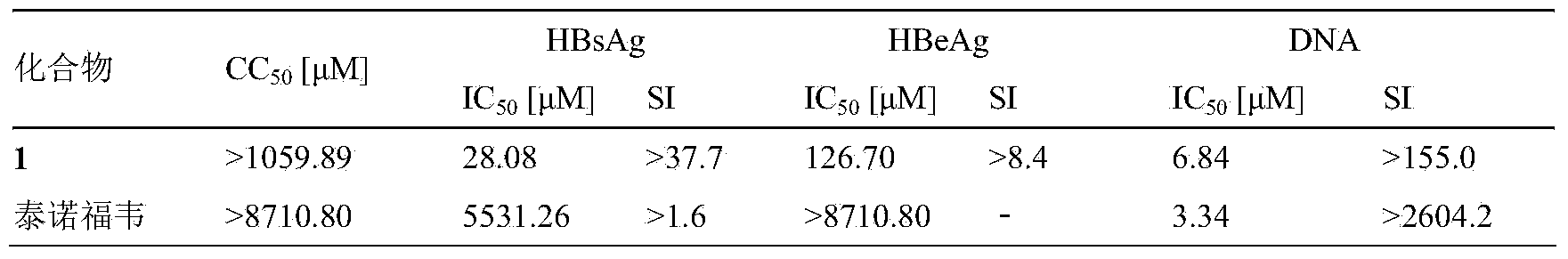
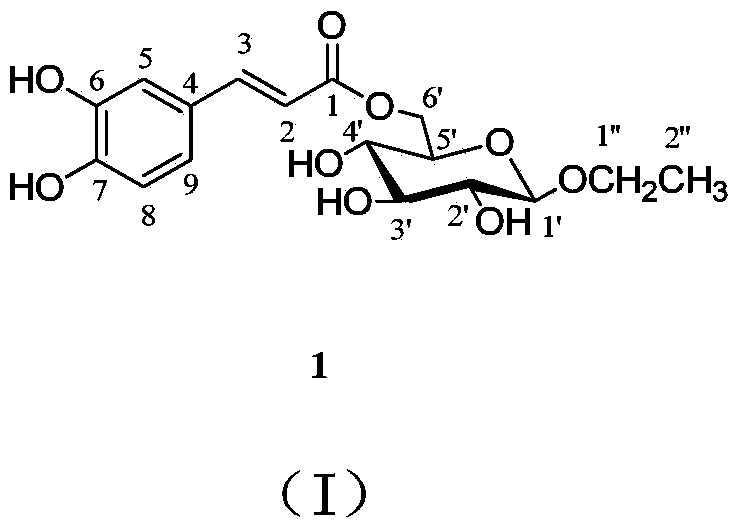
1-O-ethyl-6-O-caffeoyl-beta-D-glucopyranose,1 and pharmaceutical composition and application thereof
A technology of caffeoyl and composition, applied in the direction of preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problem of unclear anti-HBV active substances, no reports of pharmaceutical compositions, and no pharmaceutical composition B virus Hepatitis drug application reports and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of 1-O-ethyl-6-O-caffeoyl-β-D-glucopyranoside (1):
[0055] Extraction and separation of 1-O-ethyl-6-O-caffeoyl-β-D-glucopyranoside (1):
[0056] Collect the whole herb of Artemisia capillaris (10kg), whose scientific name is identified as Artemisia capillarisThunb., reflux extraction with 90% ethanol twice for 3 hours each time, combine the ethanol extracts, and recover the ethanol under reduced pressure until there is no alcohol smell. The extract was suspended in an aqueous solution, and extracted with ethyl acetate. The ethyl acetate part was dissolved and adsorbed on silica gel with methanol-chloroform, and the solvent was evaporated at room temperature. After being ground, it was subjected to silica gel column chromatography and eluted with methanol-chloroform (0:100-60:40) to obtain 7 components (A-G). Component F was prepared by medium pressure on MCI CHP-20P gel column, eluted with methanol-water (30:70-100:0), and 4 fractions F-1~F-4.F-3 were obt...
Embodiment 2
[0070] According to the method of Example 1, 1-O-ethyl-6-O-caffeoyl-β-D-glucopyranoside (1) was first prepared, after dissolving with a small amount of DMSO, water for injection was added as usual, finely filtered, Potting and sterilizing to make injection solution.
Embodiment 3
[0072] According to the method of Example 1, 1-O-ethyl-6-O-caffeoyl-β-D-glucopyranoside (1) was first prepared, and after dissolving with a small amount of DMSO, it was dissolved in sterile water for injection , stir to dissolve, filter with a sterile suction filter funnel, then aseptic fine filter, pack in ampoules, lyophilize at low temperature, and aseptically melt seal to obtain a powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com