Delta<13(18)>-CDDO derivative, its preparation method and its use in pharmacy
A compound and generative technology, applied in antineoplastic drugs, drug combinations, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
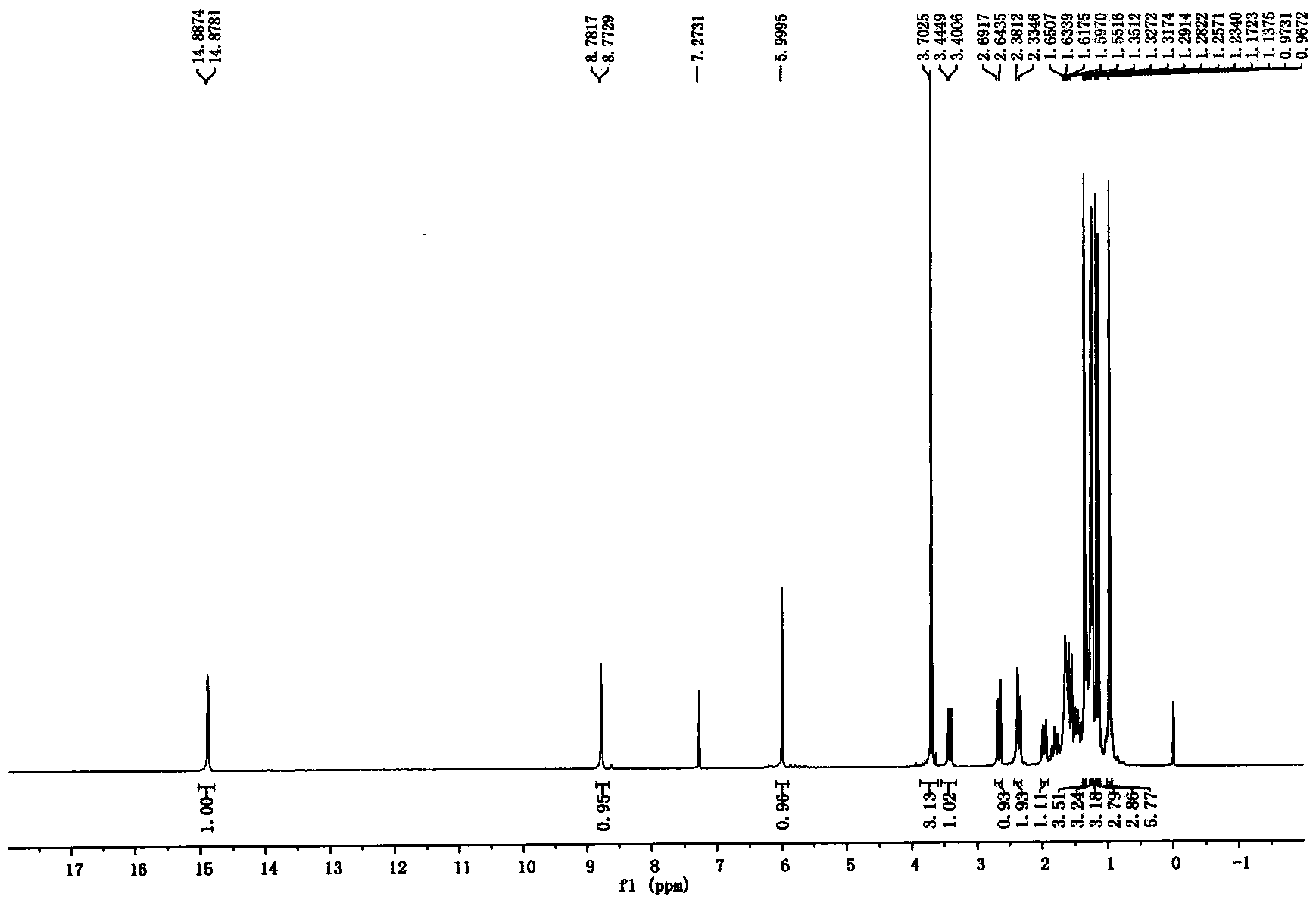
Examples
Embodiment 1
[0068] 3β-Hydroxyoleanane-12-ene-28-carboxylic acid methyl ester
[0069] Oleanolic acid (20 g, 0.043 mol) and potassium carbonate (15.1 g, 0.107 mol) were suspended in 200 mL of DMF, methyl iodide (2.9 mL, 0.047 mol) was added at room temperature, and stirred overnight. The reaction solution was filtered, the filter cake was washed with DMF, the combined reaction solution and washing solution were added to ice water, a large amount of white solids were precipitated, left to stand until the particles became larger, filtered, the filter cake was fully washed with water, dried to obtain a white solid (20g , 96%). 1 H NMR (300MHz, CDCl 3 )δ5.30(1H, t, J=3.5Hz), 3.64(3H, s), 3.23(1H, m), 2.88(1H, dd, J=4.3, 11.8Hz), 0.75(3H, s), 0.80(3H,s), 0.92(3H,s), 0.93(3H,s), 0.95(3H,s), 1.01(3H,s), 1.15(3H,s)ppm.
Embodiment 2
[0071] 3β-Hydroxyoleanane-13(18)-ene-28-carboxylic acid methyl ester
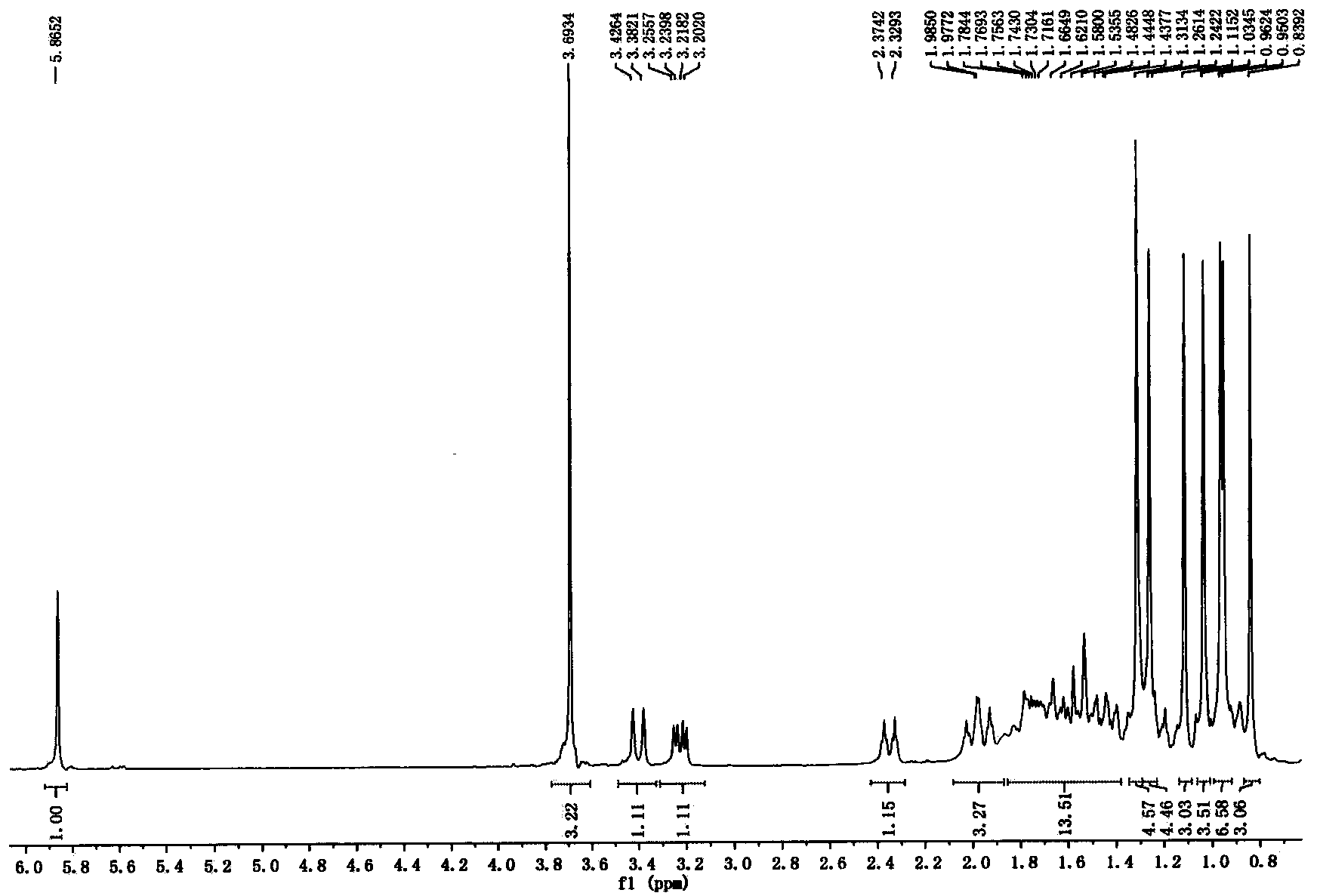
[0072] Methyl oleanolic acid (20 g, 0.041 mol) was dissolved in 500 mL of dichloromethane, and 30 g of H-MontK was added 10 , stirring under reflux for 72 hours until the end of the reaction. The reaction liquid was filtered, and the filter cake was washed with dichloromethane. The reaction liquid and the washing liquid were combined and spin-dried to obtain a white solid (18.7 g, 93.5%). 1 H NMR (300MHz, CDCl 3 ): δ0.74(3H, s), 0.77(3H, s), 0.88(3H, s), 0.906(3H, s), 0.909(3H, s), 0.99(3H, s), 1.16(3H, s), 1.86-1.89(2H, m), 2.18(1H, d, J=7.5Hz), 2.43(1H, d, J=8.4Hz), 2.73-2.77(1H, m), 3.23(1H, m ), 3.66 (3H, s) ppm.
Embodiment 3
[0074] 3β-Acetoxyoleanane-13(18)-ene-28-carboxylic acid methyl ester
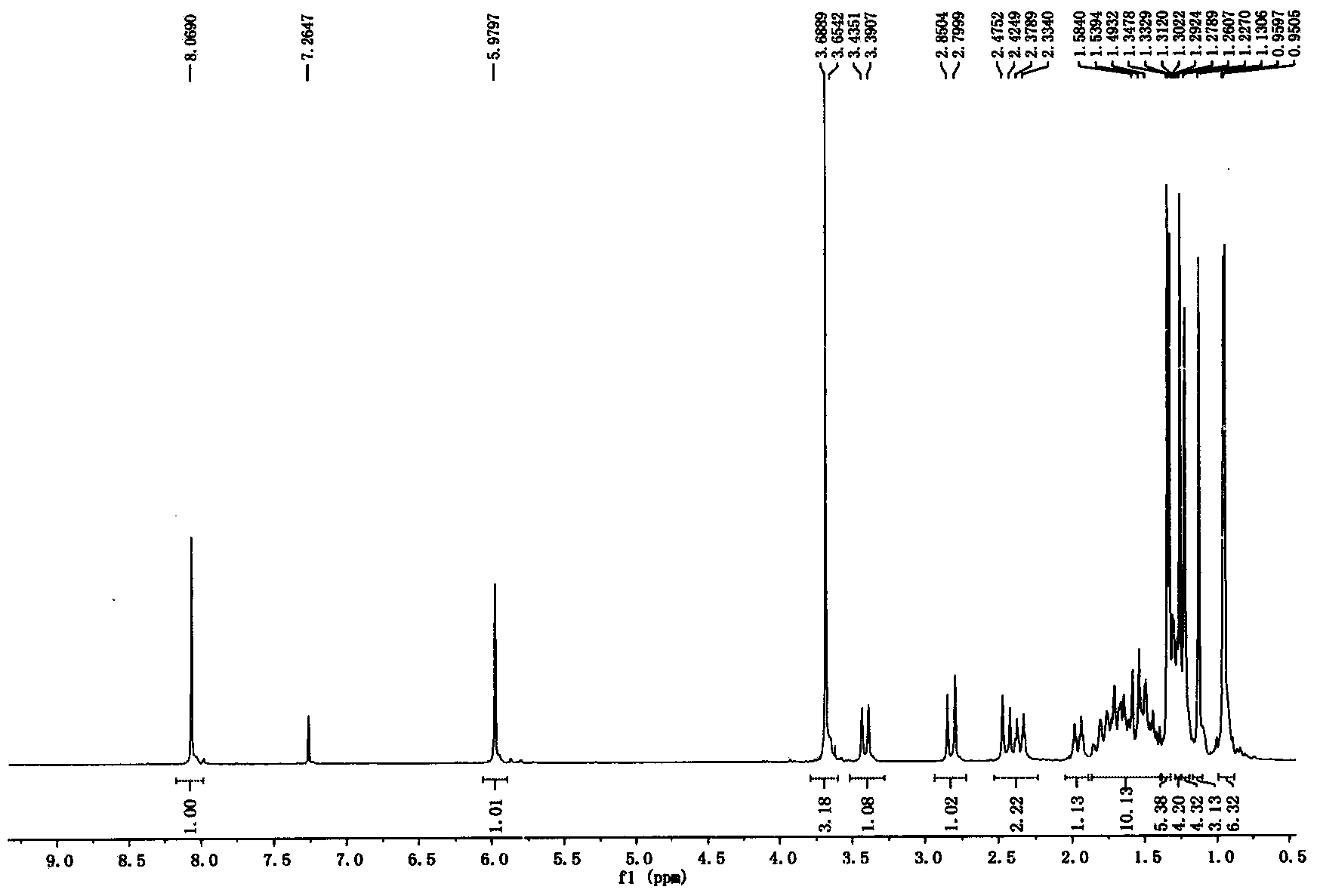
[0075] Dissolve δ-methyl oleanate (18 g, 0.037 mol) in 200 mL of dichloromethane, add acetyl chloride (3.9 mL, 0.055 mol) and a catalytic amount of triethylamine, and stir overnight at room temperature. The reaction solution was concentrated, and flash silica gel column chromatography (petroleum ether: ethyl acetate = 100:1) gave a white solid (16.2 g, 85%). 1 HNMR (500MHz, CDCl 3 ): δ4.50(1H, m), 3.64(3H, s), 2.73(1H, m), 2.40(1H, d, J=14.05Hz), 2.16(1H, m), 2.03(3H, s) , 1.14(3H, s), 0.90(9H, s), 0.85(3H, s), 0.83(3H, s), 0.72(3H, s)ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com