Orally-taken recombined fusion protein TAT-MAP30, preparation method and applications
A fusion protein and protein technology, which is applied in the direction of recombinant DNA technology, biochemical equipment and methods, peptide/protein components, etc., can solve the difficult and impossible problems of controlling and treating animal viral diseases, and achieve low cost, The effect of large amount of expression and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
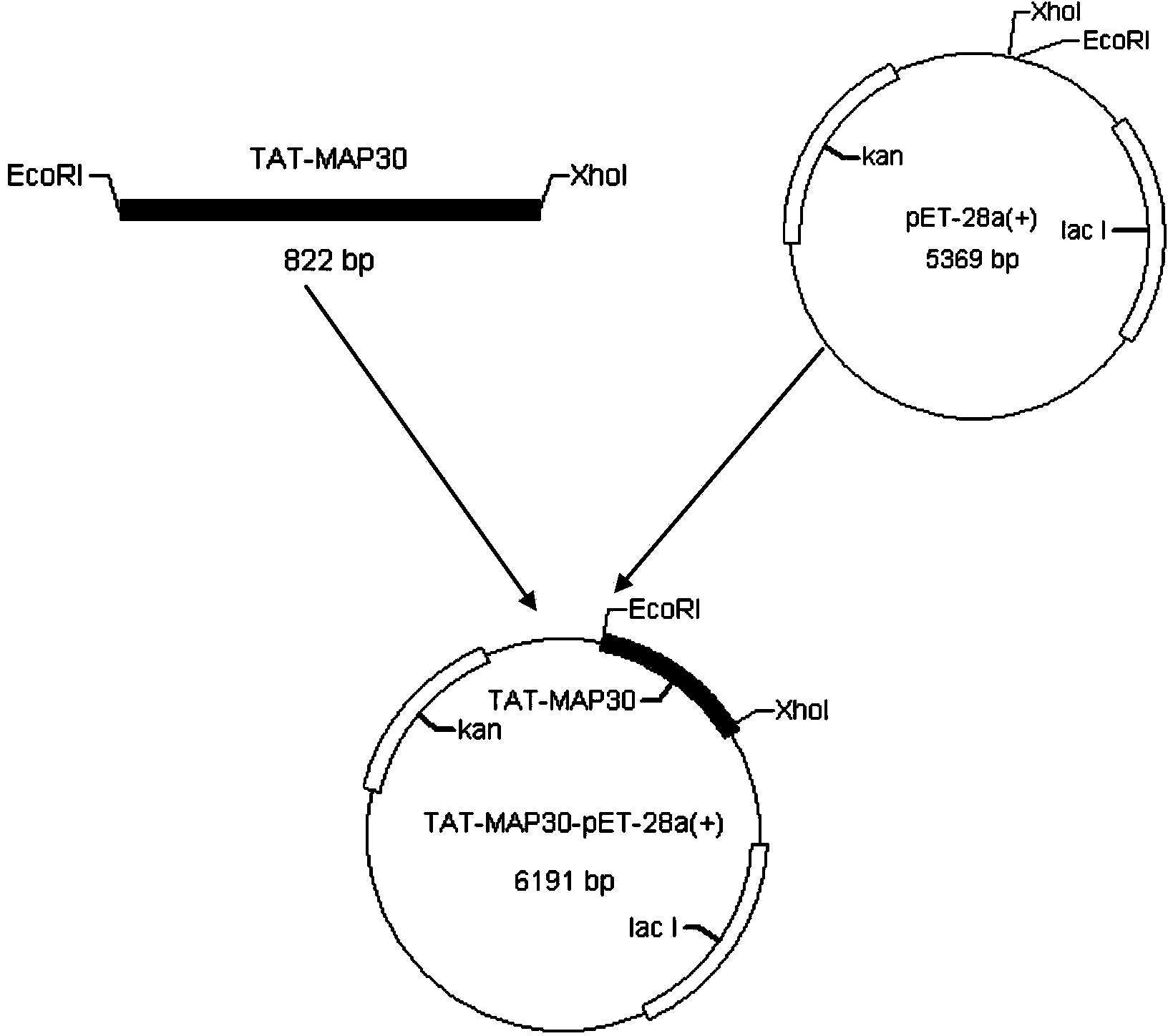
[0085] Preparation of engineering bacteria Escherichia coli BL21 (DE3) pET-28a-TAT-MAP30
[0086] 1. Obtaining of MAP30 gene and TAT sequence
[0087] The sequence of the artificially synthesized MAP30 gene is shown in SEQ ID NO.3.
[0088] TAT sequence according to Dietz GP et al. Application of a blood-brain-barrier-penetrating form of GDNF in a mouse model for Parkinson's disease. Brain Res .2006Apr12;1082(1):61-6. According to literature reports, a biological company was entrusted to synthesize the full sequence of the TAT gene and save it on the T vector. The TAT sequence is shown in SEQ ID NO.4.
[0089] 2. Acquisition of TAT-MAP30 fusion gene
[0090] Using fusion PCR technology, a TAT sequence was added to the 3' end of the MAP30 gene sequence to realize the fusion of two gene sequences. In order to add TAT at the MAP305' end by overlapping PCR technology, the primers are now designed as follows:
[0091]
[0092]
[0093] Explanation: The bolded part is ...
Embodiment 2
[0145] Obtaining of Recombinant Fusion Protein TAT--MAP30
[0146] 1. Identification of expression of recombinant fusion protein TAT--MAP30
[0147] The engineered strain Escherichia coli BL21 (DE3) pET-28a-TAT-MAP30 was induced by IPTG, and identified by SDS-PAGE gel and Western-blotting, which proved that the recombinant fusion protein TAT-MAP30 was effectively expressed. The specific method is as follows:
[0148] ①Inoculate 50uL of Escherichia coli BL21(DE3)pET-28a-TAT-MAP30 glycerol bacterium in 20mL LB liquid medium and culture overnight.
[0149] ②According to the proportion of 4%, inoculate 800uL overnight culture into 20ml rich medium (2×YT), and culture at 37℃200rpm until OD600 is about 0.6.
[0150] ③ Place the flask in ice bath for 10-20 minutes.
[0151] ④Take 1ML of the culture as a negative control, add 10ul of IPTG (the concentration of the mother solution is 1mol / ml) to the remaining culture medium, and incubate at 20°C and 200rpm for 12-18 hours.
[0152]...
Embodiment 3
[0182] ELISA detection of TAT-MAP30 protein's intestinal penetration function
[0183] 1. Enzyme-linked immunosorbent assay (ELISA) related solution
[0184] Chromogenic solution:
[0185] Weigh 0.47g citric acid, 0.92g Na 2 HPO 4 -12H 2 O, dilute to 100ml, which is prepared as substrate buffer. Just before use, add 40mg o-phenylenediamine, 0.1ml H 2 o 2 , mix well.
[0186] Stop solution: 2mol / L H 2 SO 4 .
[0187] PBS buffer:
[0188] Weigh 8.5g NaCl, 2.85g Na 2 HPO 4 -12H 2 O, 0.2g KCl, 0.27g KH 2 PO 4 , add deionized water to make the volume to 1000ml.
[0189] PBST buffer:
[0190] Add 0.5-2ml Tween-20 to 1000ml PBS buffer.
[0191] 5%BSA:
[0192] Weigh 0.5g BSA and dissolve in 10ml PBST buffer.
[0193] 2. Experimental materials
[0194] The Crayfish (Cambarus clarkii) used in the experiment was purchased from Wuhan Aquatic Products Market, with a weight of about 15-20 g. After the purchase, it was raised in our laboratory at a temperature of about ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mean radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com