Biology activity determination method of monoclonal antibody
A monoclonal antibody and biological activity technology, which is applied in the field of antibody biological activity determination, can solve the problems of poor applicability of anti-EGFR antibodies, increased variation detection results, and low sensitivity, so as to achieve more controllable quality, Wide applicability and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
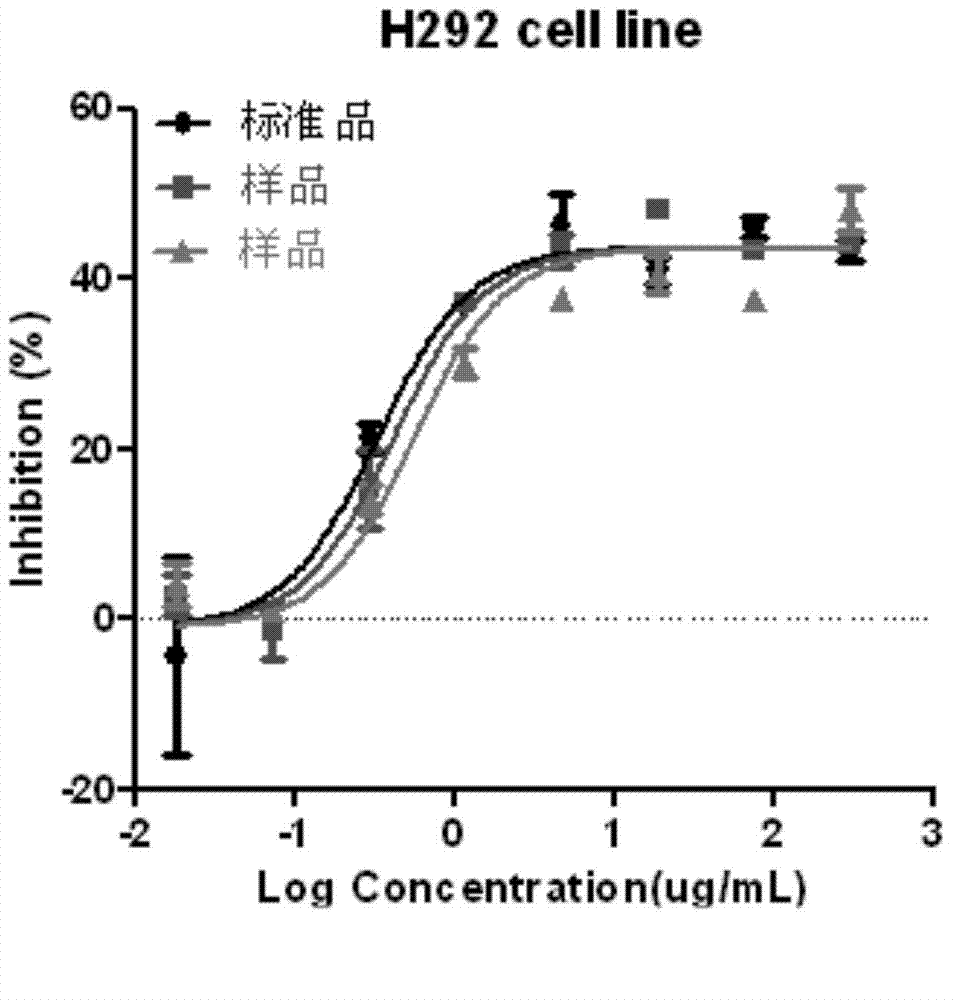
[0035] Example 1: Determination of biological activity of recombinant anti-human epidermal growth factor receptor monoclonal antibody by in vitro proliferation inhibition method
[0036] The method for measuring the activity of anti-EGFR antibody using human lung cancer lymph node metastasis cell line H292 (purchased from Cuba Molecular Immunology Center) includes the following steps:
[0037] (1) Preparation of H292 cell suspension: The cell viability of the H292 cell suspension should not be lower than 85%, and the cells should be counted. In a clean bench, use complete culture medium to make a density of (6-10)×10 4 cells / mL of cell suspension.
[0038] (2) The cell suspension was inoculated in a 96-well plate, 100 μL / well (blank control well plus 100 μL complete culture solution) was placed at 37°C CO 2 cultured in an incubator.
[0039] (3) Prepare Nimotuzumab solutions of different concentrations, dilute the Nimotuzumab solution samples or standards to (300-1000) μg / m...
Embodiment 2
[0051] Example 2: Method verification for measuring the biological activity of anti-EGFR monoclonal antibody by in vitro cell proliferation inhibition method
[0052] The verification of the method for determining the biological activity of nimotuzumab using the H292 cell line of lung cancer lymph node metastasis (purchased from the Cuban Molecular Immunology Center) includes the following steps:
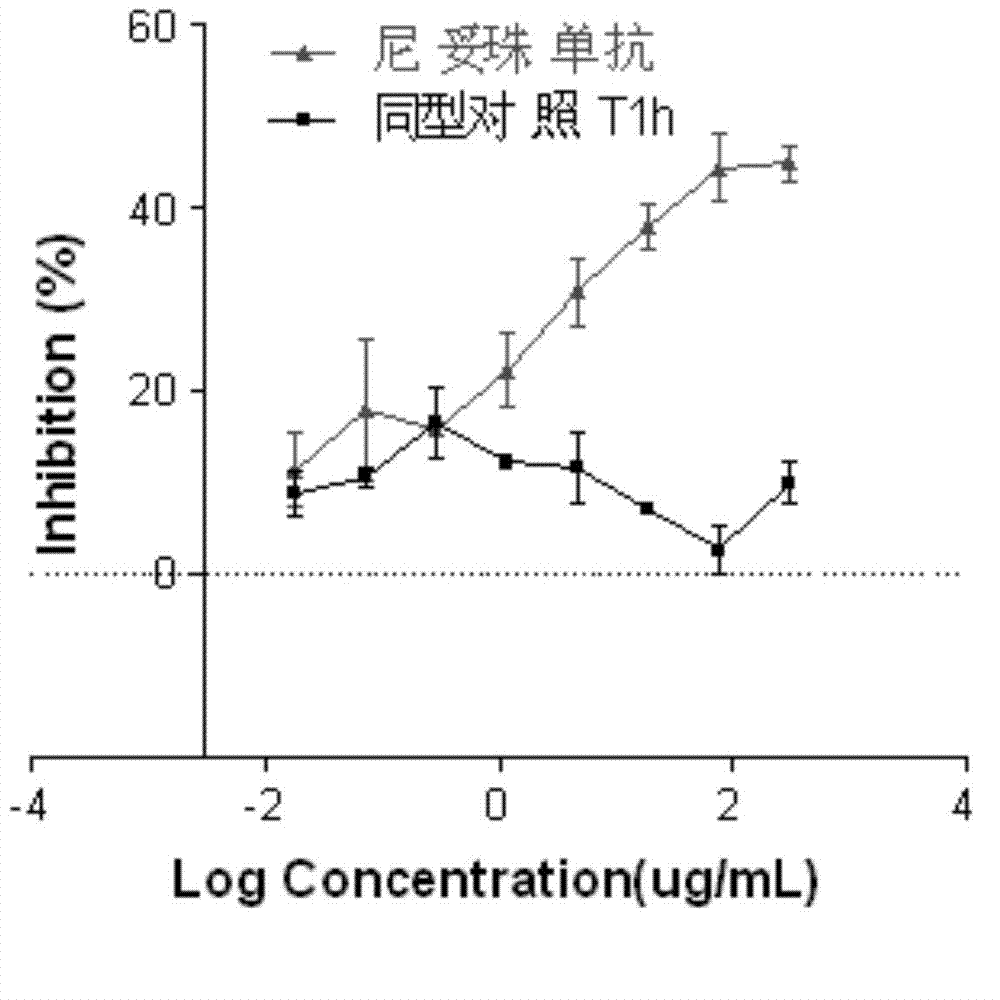
[0053] (1) Specificity: Nimotuzumab is a human IgG1 subclass kappa immunoglobulin, and the same human IgG1 subclass kappa immunoglobulin was used as the isotype control, and the experiment was repeated at least twice. Acceptable standard: the isotype non-EGFR monoclonal antibody should have almost no cross-reaction with H292 cells, and should not show a similar response curve.
[0054] The result is as figure 2 As shown, the isotype control antibody T1h monoclonal antibody has almost no cross-reaction with H292 cells, and does not show a similar reaction curve, indicating that T1h...
Embodiment 3
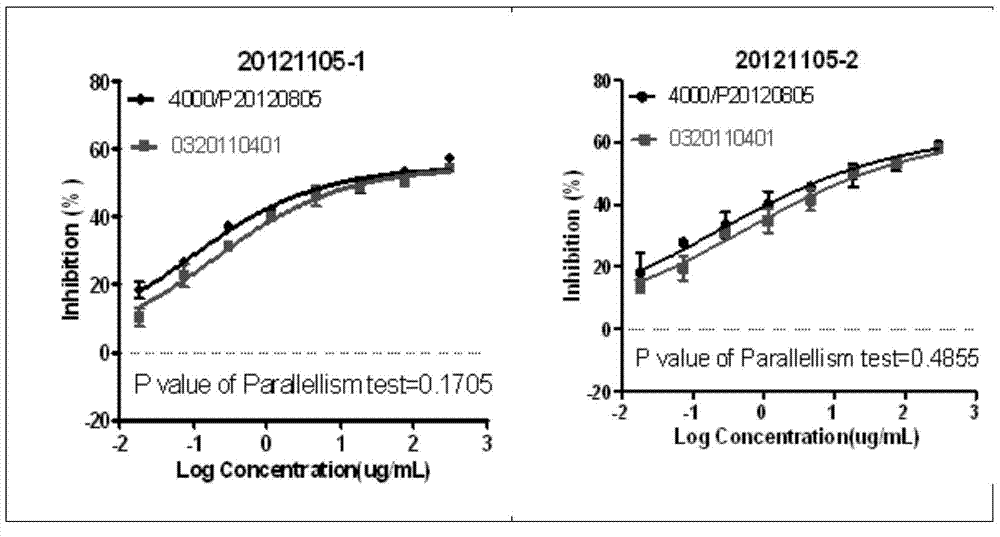
[0068] Example 3: Biological Activity Comparison of Different Recombinant Anti-EGFR Monoclonal Antibodies
[0069] High affinity antibody: 10 affinity to EGFR -10 ~10 -11
[0070] Medium affinity antibody: 10 affinity to EGFR -8 ~10 -9
[0071] The high-affinity antibody used in this example is cetuximab, and the medium-affinity antibody is nimotuzumab. Carry out anti-EGFR monoclonal antibody biological activity assay according to the step of embodiment 1, test data is shown in the following table:
[0072]
[0073] From the data in the table above, the IC of the high-affinity antibody 50 The value is about 0.0002611, the IC of a medium affinity antibody 50The value is 1.634, indicating that the H292 cell line is sensitive to antibodies with different affinities, and is especially suitable for the determination of the biological activity of antibodies with low to medium affinity. Commonly used cell lines such as MB-468 cell line are not sensitive. IC of Hi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com