Broom-shaped hydroxycamptotecin-loaded sustained-release particle and preparation method thereof
A technology for carrying hydroxyl and camptothecin, which can be used in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., and can solve the problems of increasing adverse reactions, easy decomposition, and reducing efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
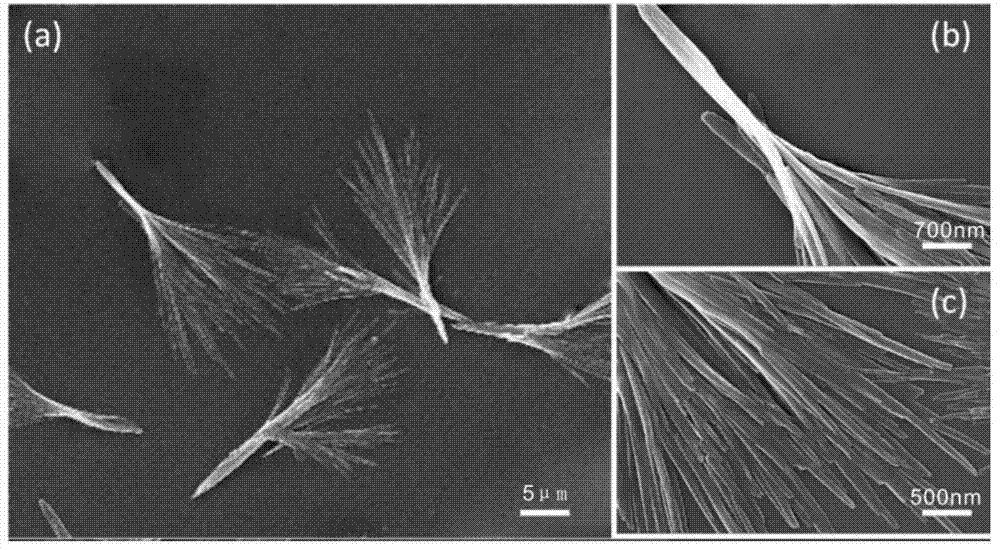
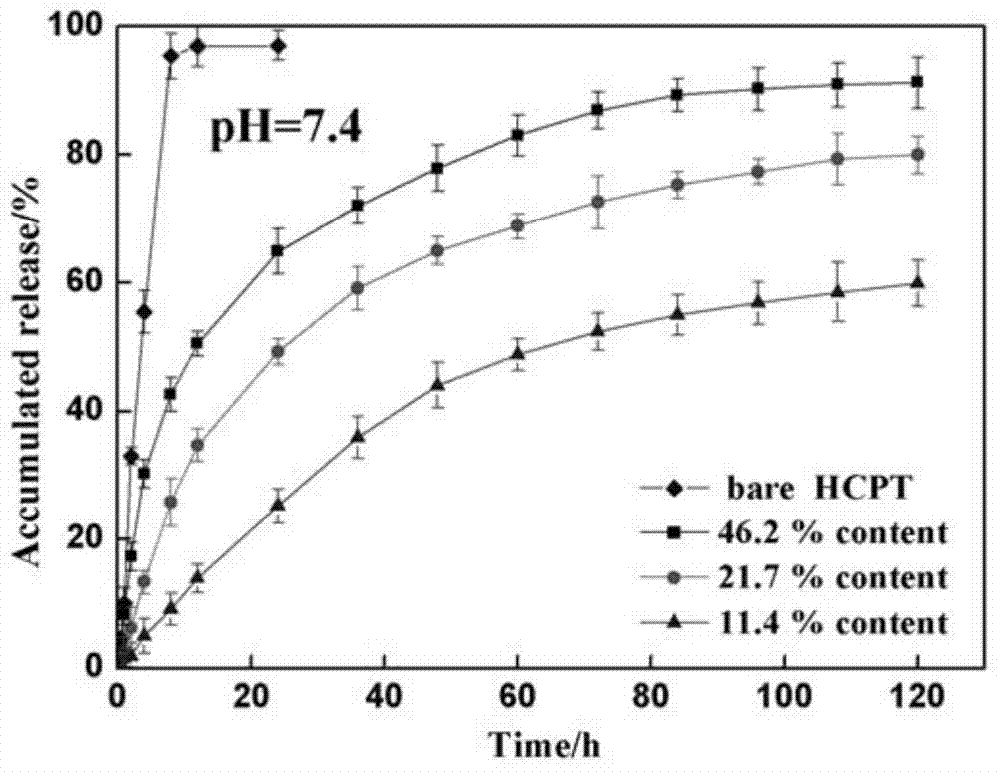
[0013] After co-dissolving 20mg of 10-HCPT and 20mg of MePEG-PLGA in 80mL of acetone, inject it into the dispersed phase inlet of the membrane emulsifier, and inject the aqueous solution containing 0.25% PVA into the continuous phase of the membrane emulsifier, using 1.1 μm SPG membrane as a template, in Under the pressure of 100KPa pure nitrogen, the dispersed phase solution quickly enters the continuous phase to obtain a MePEG-PLGA-HCPT suspension. After the above suspension is vacuum rotary evaporated to remove acetone, it is filtered with a 1 μm microporous membrane, and then the obtained suspension is The solution was vacuum freeze-dried in a freeze dryer for 24 hours to obtain MePEG-PLGA-HCPT powder with a drug loading of 38.3%. figure 1 The scanning electron micrographs of the nanoparticles prepared in Example 1 are given.
Embodiment 2
[0015] After co-dissolving 20mg of 10-HCPT and 20mg of MePEG-PLGA in 80mL of acetone, inject it into the dispersed phase feed port of the membrane emulsifier, and inject the aqueous solution containing 0.25% PVA into the continuous phase of the membrane emulsifier, using 0.6μm SPG membrane as a template, in Under the pressure of 100KPa pure nitrogen, the dispersed phase solution quickly enters the continuous phase to obtain a MePEG-PLGA-HCPT suspension. After the above suspension is vacuum rotary evaporated to remove acetone, it is filtered with a 1 μm microporous membrane, and then the obtained suspension is The solution was vacuum freeze-dried in a freeze dryer for 24 hours to obtain MePEG-PLGA-HCPT powder with a drug loading of 32.7%.
Embodiment 3
[0017] After co-dissolving 20mg of 10-HCPT and 40mg of MePEG-PLGA in 80mL of acetone, inject it into the dispersed phase inlet of the membrane emulsifier, and inject ultrapure water containing 0.25% PVA into the continuous phase of the membrane emulsifier, using a 0.4μm SPG membrane as a template , under the pressure of 50KPa pure nitrogen, the dispersed phase solution quickly enters the continuous phase to obtain the MePEG-PLGA-HCPT suspension, the above suspension is vacuum rotary evaporated to remove acetone, filtered with a 1 μm microporous membrane, and the resulting mixture The suspension was vacuum freeze-dried in a freeze dryer for 24 hours to obtain MePEG-PLGA-HCPT powder with a drug loading of 21.4%.
[0018] Example 3
[0019] After co-dissolving 20mg10-HCPT and 40mg MePEG-PLGA in 80mL acetone, inject it into the dispersed phase feed port of the membrane emulsifier, and inject the aqueous solution containing 0.25% PVA into the continuous phase of the membrane emulsi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com