Catalyst precursor and its preparation method, catalyst and its application, and ethylene polymerization method
A technology of catalysts and bimetallic catalysts, applied in chemical instruments and methods, titanium organic compounds, compounds of group 4/14 elements of the periodic table, etc., can solve problems such as complex catalyst preparation processes, and achieve simple structure and high catalytic efficiency , Improve the effect of polymerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
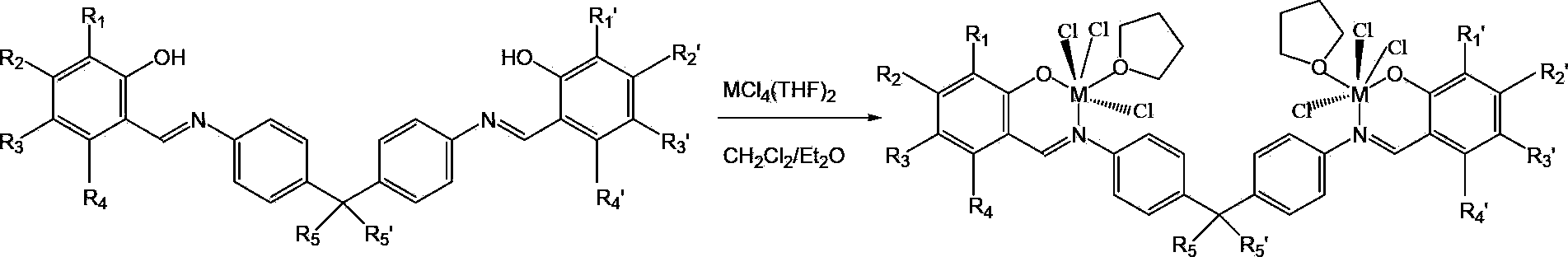
[0035] The present invention also provides a method for preparing the catalyst precursor, wherein the method includes under the conditions of the complexation reaction, the method includes: the method includes under the conditions of the complexation reaction, will have the structure shown in formula IV The general formula of compound and molecular structure is MCl 4 (THF) 2 The compound is contacted in an organic solvent to obtain the above-mentioned bimetallic catalyst precursor with an open structure shown in formula I based on a salicylaldimine ligand.
[0036] Formula IV,
[0037] Among them, R 1 , R 2 , R 3 , R 4 , R 1 ’, R 2’, R 3 ’ and R 4 ' each independently hydrogen, phenyl or C1-C20 alkyl, R 5 and R 5 ' each independently hydrogen or C1-C20 alkyl;
[0038] M is one of titanium, zirconium and hafnium, preferably titanium.
[0039] The preparation process of the above-mentioned bimetallic catalyst precursor with the open structure shown in formula I ba...
preparation example 1
[0066] This preparation example is used to illustrate the preparation of the bis salicylaldimine-titanium metal catalyst precursor with the structure shown in formula II.
[0067] 6,6'-(1E,1'E)-(4,4'-methylenebis(4,1-phenylene)bis(imine-1-substituted-1-ylidene))bis(form Base-1-substituted-1-ylidene)bis(2-tert-butylphenol) (prepared according to the preparation method recorded in Eur.Polym.J.2012,48,191-199, the same below) (1.77g, 3.41mmol ) was dissolved in dichloromethane solvent (the amount of dichloromethane used was 30mL), and the solution was added to the dichloromethane solution containing tetrachlorobis(tetrahydrofuran)titanium (2.28g, 6.82mmol) at -78°C (the amount of dichloromethane used is 30 mL), react at low temperature for 1 hour, return to room temperature 25°C, and continue to react for 16 hours. After the reaction was finished, the solvent was removed with a vacuum line, the residue was washed with dichloromethane and filtered through diatomaceous earth, the ...
preparation example 2
[0070] This preparation example is used to illustrate the preparation of the bis salicylaldimine-titanium metal catalyst precursor with the structure shown in formula II.
[0071]6,6'-(1E,1'E)-(4,4'-methylenebis(4,1-phenylene)bis(imine-1-substituted-1-ylidene))bis(form Base-1-substituted-1-ylidene)bis(2-tert-butylphenol)(0.24g, 0.46mmol) was dissolved in dichloromethane solvent (the amount of dichloromethane used was 30mL), and the Add this solution to a dichloromethane solution (the amount of dichloromethane is 30mL) containing tetrachlorobis(tetrahydrofuran)titanium (0.32g, 0.96mmol), react at low temperature for 1 hour, return to room temperature and heat to 40 °C, the reaction was continued for 12 hours. After the reaction was finished, the solvent was removed with a vacuum line, the residue was washed with dichloromethane and filtered through diatomaceous earth, the filtrate was sucked dry, and the crude product was recrystallized with dichloromethane / n-hexane to obtain ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com