Gel polymer electrolyte and preparation method thereof
A gel polymer and electrolyte technology, applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., can solve the problems of low conductivity and low lithium ion migration number of gel polymer electrolytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
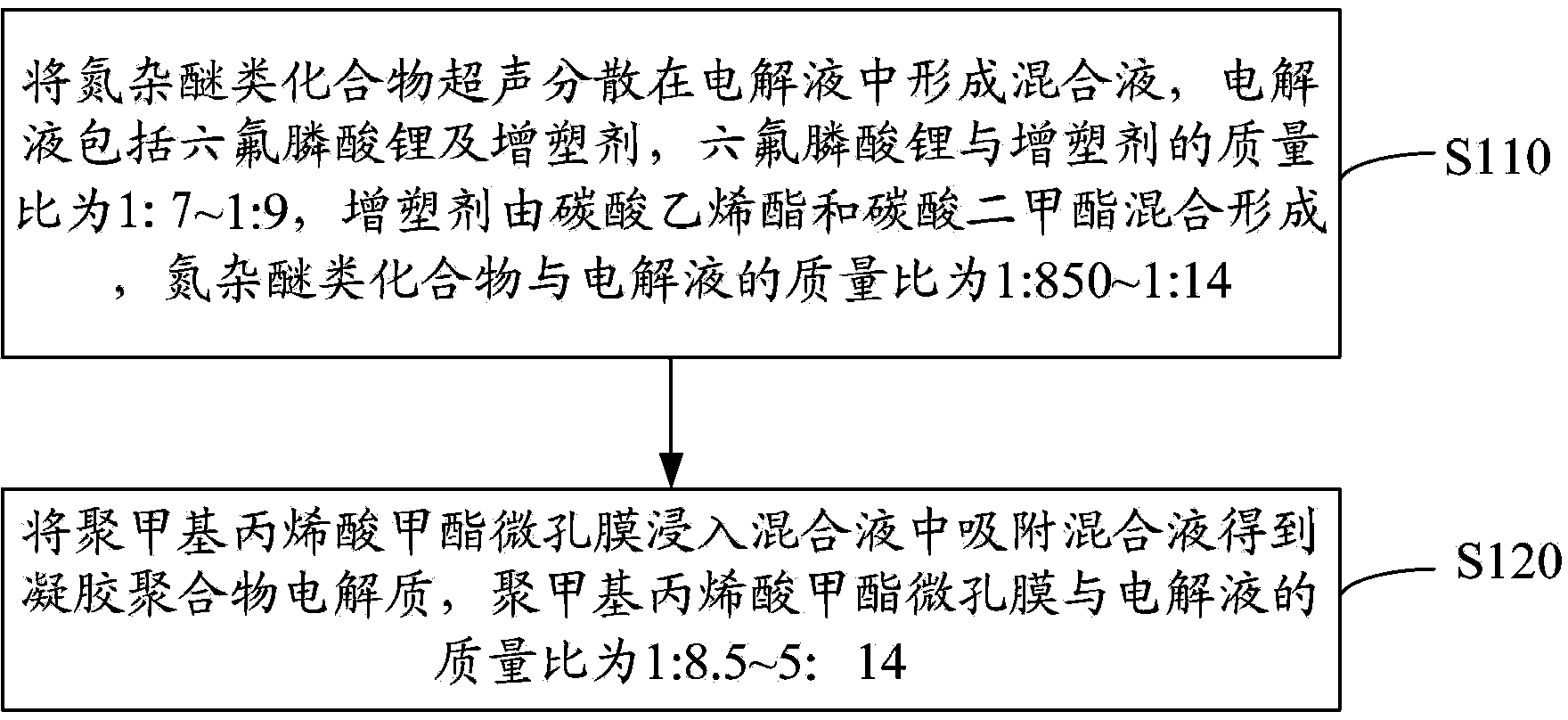
[0024] see figure 1 , the preparation method of above-mentioned gel polymer electrolyte, comprises the following steps:
[0025] Step S110, ultrasonically dispersing the azaether compound in the electrolytic solution to form a mixed solution, the electrolytic solution includes lithium hexafluorophosphonate and a plasticizer, and the mass ratio of lithium hexafluorophosphonate to the plasticizer is 1:7-1 :9, the plasticizer is formed by mixing ethylene carbonate and dimethyl carbonate, and the mass ratio of azaether compound to electrolyte is 1:850~1:14.
[0026] Preferably, the azaether compound is selected from three (hexafluoroisopropoxy) amines ([(CF 3 ) 2 CHO] 3 N) and three perfluorophenoxyamines ((C 6 f 5 O) 3 One of N).
[0027] Preferably, the volume ratio of ethylene carbonate to dimethyl carbonate is 1:1.5˜1:3.
[0028] Preferably, the time for ultrasonic dispersion is 5 minutes to 30 minutes.
[0029] Step S120, immersing the polymethyl methacrylate micropo...
Embodiment 1
[0035] In a glove box, ultrasonically disperse 0.5g of tris(hexafluoroisopropoxy)amine (30min) in 8.5g of electrolyte to form a mixed solution, wherein the electrolyte includes LiPF 6 and plasticizer, LiPF 6 The mass ratio of the plasticizer to the plasticizer is 1:8, and the plasticizer is formed by mixing EC and DMC with a volume ratio of 1:2, and then immersing 1g of the dried PMMA microporous membrane in the above mixture, and after 180min, the PMMA microporous membrane The membrane completely absorbs the mixed solution to obtain a PMMA-based gel polymer electrolyte added with tris(hexafluoroisopropoxy)amine. In the obtained gel polymer electrolyte, the mass percentage of PMMA matrix is 10%, the mass percentage of lithium salt and plasticizer is 85%, and the mass percentage of azaether compound is 5%.
Embodiment 2
[0037] In the glove box, ultrasonically disperse 0.4g trisperfluorophenoxyamine (ultrasonic 25min) in 8.4g electrolyte to form a mixed solution, wherein the electrolyte includes LiPF 6 and plasticizer, LiPF 6 The mass ratio with the plasticizer is 1:9, and the plasticizer is formed by mixing EC and DMC with a volume ratio of 1:1.5, and then immersing 1.2g of dried PMMA microporous membrane in the above mixed solution, and after 150min, it is Take it out to obtain the PMMA-based gel polymer electrolyte added with triperfluorophenoxyamine. In the obtained gel polymer electrolyte, the mass percentage of PMMA matrix is 12%, the mass percentage of lithium salt and plasticizer is 84%, and the mass percentage of azaether compound is 4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com