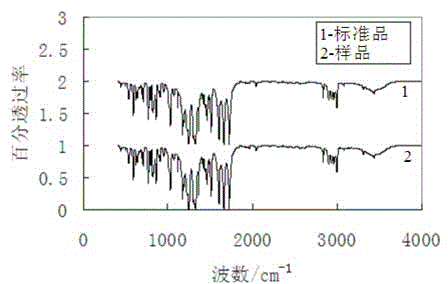
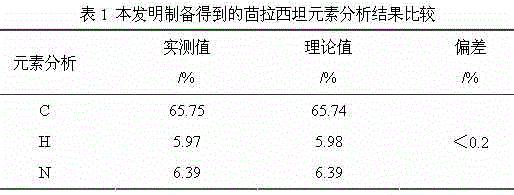
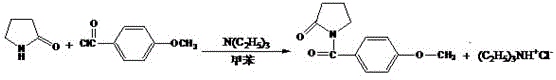Synthesis method of aniracetam
A synthetic method, the technology of aniracetam, which is applied in the field of drug synthesis, can solve the problems of using dangerous solvents and cumbersome operations, and achieve the effects of safe production, high yield, and maximized economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Preparation of aniracetam: 17.0 g (0.2 mol) of α-pyrrolidone and 40.0 mL (0.28 mol) of triethylamine were dissolved in 100.0 mL of toluene. Control the temperature below 50°C and add dropwise 40 mL of toluene solution in which 34.2 g (0.2 mol) of p-methoxybenzoyl chloride is dissolved. After dripping, the water bath kept the temperature at 50°C for 2 hours. Pour the reaction solution into a 1000mL beaker, add 500mL of water, stir, filter with suction, and wash with water until the Cl ion of the washing solution is detected to be qualified, then dry the filter cake, and then recrystallize with ethanol to obtain white flaky crystals 37.2 g (84.7% yield). Melting point, 120.2-120.8°C.
[0015] Concentrate the above washing solution under reduced pressure (pressure less than -0.05MPa) to 1 / 4 of the volume of the original solution. The concentrated solution was lowered to room temperature, and 2 times the volume of ethanol was added for alcohol analysis to obtain 16.5 g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com