Histone deacetylase inhibitor and preparation method and application thereof
A technology of deacetylase and histone, applied in the field of biomedicine, can solve the problems of uncontrollable toxic and side effects, inability to achieve therapeutic effect of chemical drugs, and drug resistance, and achieve induction of differentiation and apoptosis, and good inhibitory activity , the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
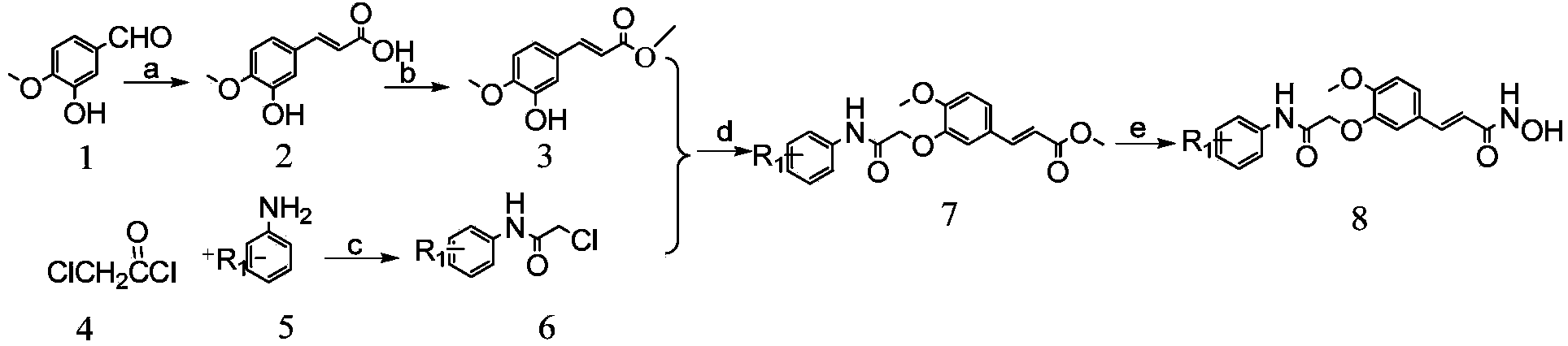
[0054] The compound is a hydroxamic acid, the R in its structural formula 1 , R 2 3,5-Ditrifluoromethyl and hydroxylamine, respectively, prepared by the following steps:
[0055] 1) Preparation of isoferulic acid from isovanillin by Knaevengel condensation reaction
[0056] Put 22.81g (150mmol) of isovanillin and 62.44g (600mmol) of malonic acid in a 500mL round bottom flask, add 150mL of anhydrous pyridine to dissolve, add 34.26g of 1,8-diazabicyclo[5.4.0] Undec-7-ene (DBU), heating to reflux reaction; after the reaction is complete, cool to room temperature, pour the reaction mixture into a mixture of 500mL of concentrated hydrochloric acid and water (V:V=1:1), A precipitate precipitated out, filtered and washed with acid water to obtain a crude product. Dissolve the crude product in 200mL of 1mol / L sodium hydroxide aqueous solution, extract with dichloromethane, adjust the pH of the aqueous solution to 1, filter the obtained precipitate, wash with acid water, and dry to ...
Embodiment 2
[0075] R in the structural formula of this compound 1 , R 2 Respectively 3-chloro-4-fluoro and hydroxylamine, the preparation method is:
[0076] The 3,5-bistrifluoromethylaniline in Example 1 was replaced with 3-chloro-4fluoromethylaniline, and the other preparation methods were the same.
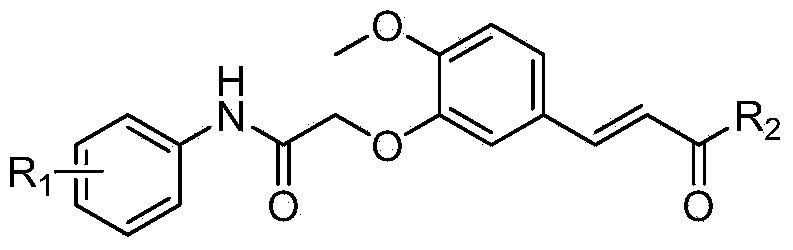
[0077] The resulting intermediate is N-(3-chloro-4-fluorophenyl)-2-{2-methoxy-5-[(1E)-3-oxobutenyl]phenoxy}acetamide, which The structure of the compound is shown below:
[0078]
[0079] Physical and chemical properties: mp: 182-184 ° C, MS (EI) [M] + :m / z=393.0.
[0080] Proton NMR data: 1 H NMR (400MHz, CDCl 3 )δppm:7.80(d,J=4Hz,1H),7.63(d,J=8Hz,1H),7.45(d,J=8Hz,1H),7.26(s,1H),7.17(s,1H), 7.14(d,J=4Hz,1H),6.98(d,J=4Hz,1H),6.34(d,J=8Hz,1H),4.68(s,2H),4.00(s,3H),3.83(s ,3H).
[0081] The target compound is (2E)-3-(5-{2-[(3-chloro-4-fluorophenyl)amino]-2-oxoethoxy}-3-methoxyphenyl)-N- Hydroxyacrylamide, the structure of the compound is shown below:
[0082]
[0083] Physic...
Embodiment 3
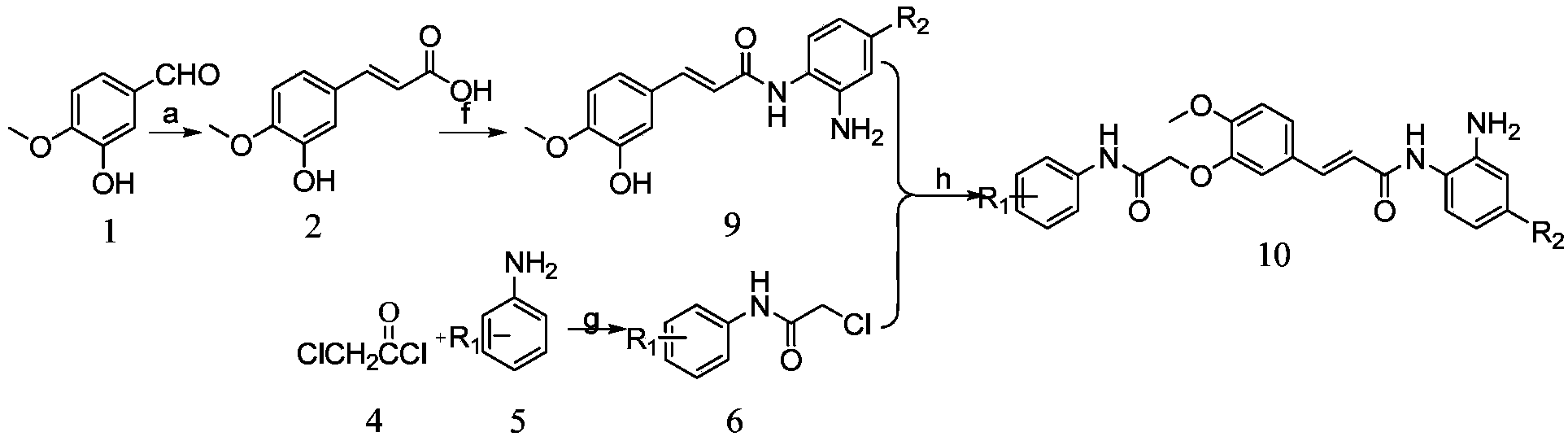
[0086] R in the structural formula of this compound 1 , R 2 3-Bromo-5-trifluoromethyl and 2-aminoaniline, respectively, prepared by:
[0087] 1) Preparation of isoferulic acid from isovanillin by Knaevengel condensation reaction
[0088]Put 22.81g (150mmol) of isovanillin and 62.44g (600mmol) of malonic acid in a 500mL round bottom flask, add 150mL of anhydrous pyridine to dissolve, add 34.26g of 1,8-diazabicyclo[5.4.0] Undec-7-ene (DBU), heating to reflux reaction; after the reaction is complete, cool to room temperature, pour the reaction mixture into a mixture of 500mL of concentrated hydrochloric acid and water (V:V=1:1), A precipitate precipitated out, filtered and washed with acid water to obtain a crude product. Dissolve the crude product in 200mL of 1mol / L sodium hydroxide aqueous solution, extract with dichloromethane, adjust the pH of the aqueous solution to 1, filter the obtained precipitate, wash with acid water, and dry to obtain 22.56g of white crystal isoferu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com