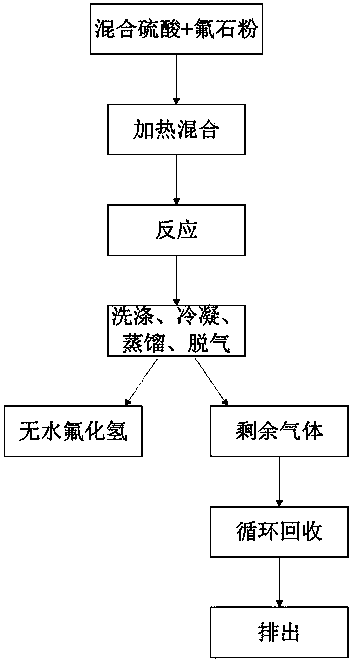
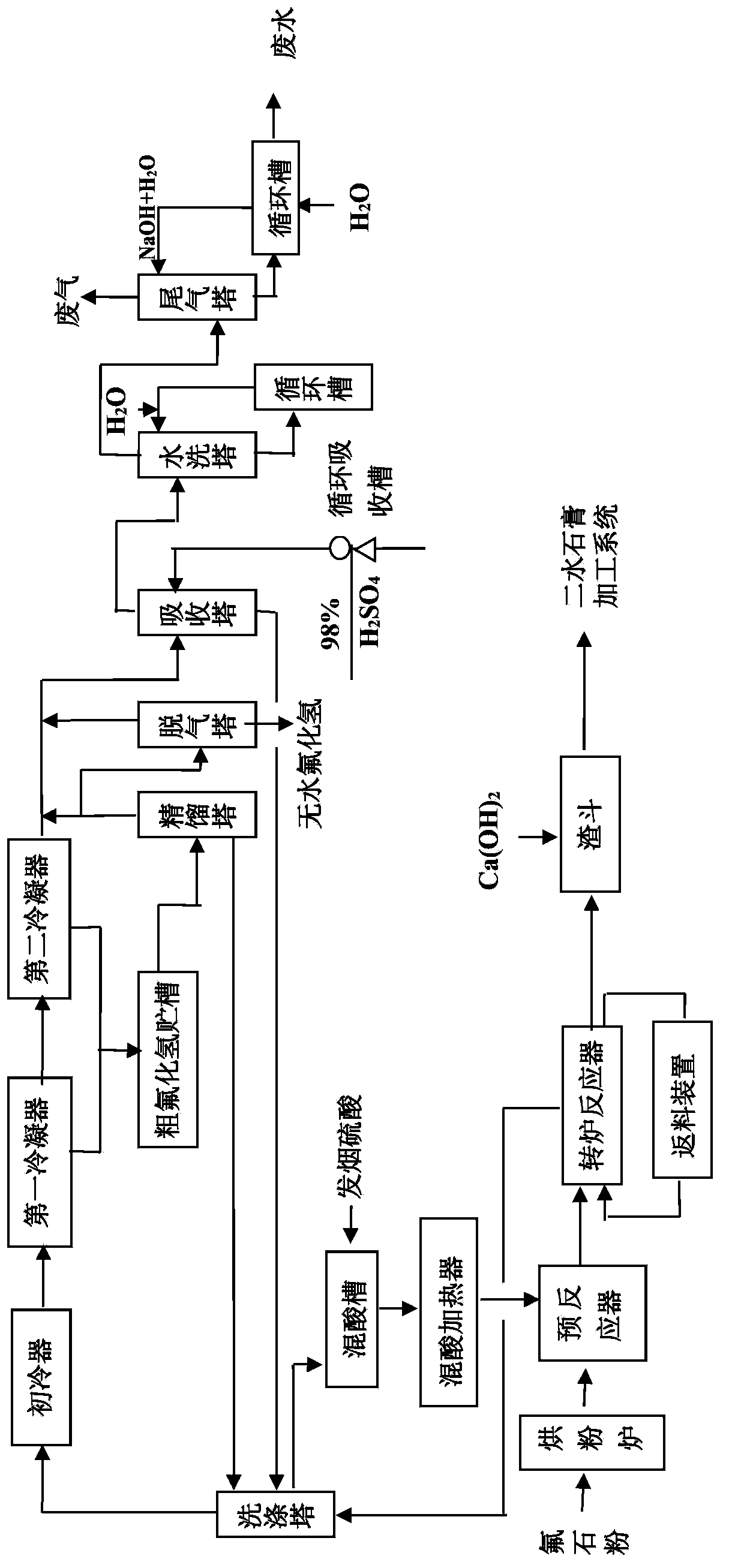
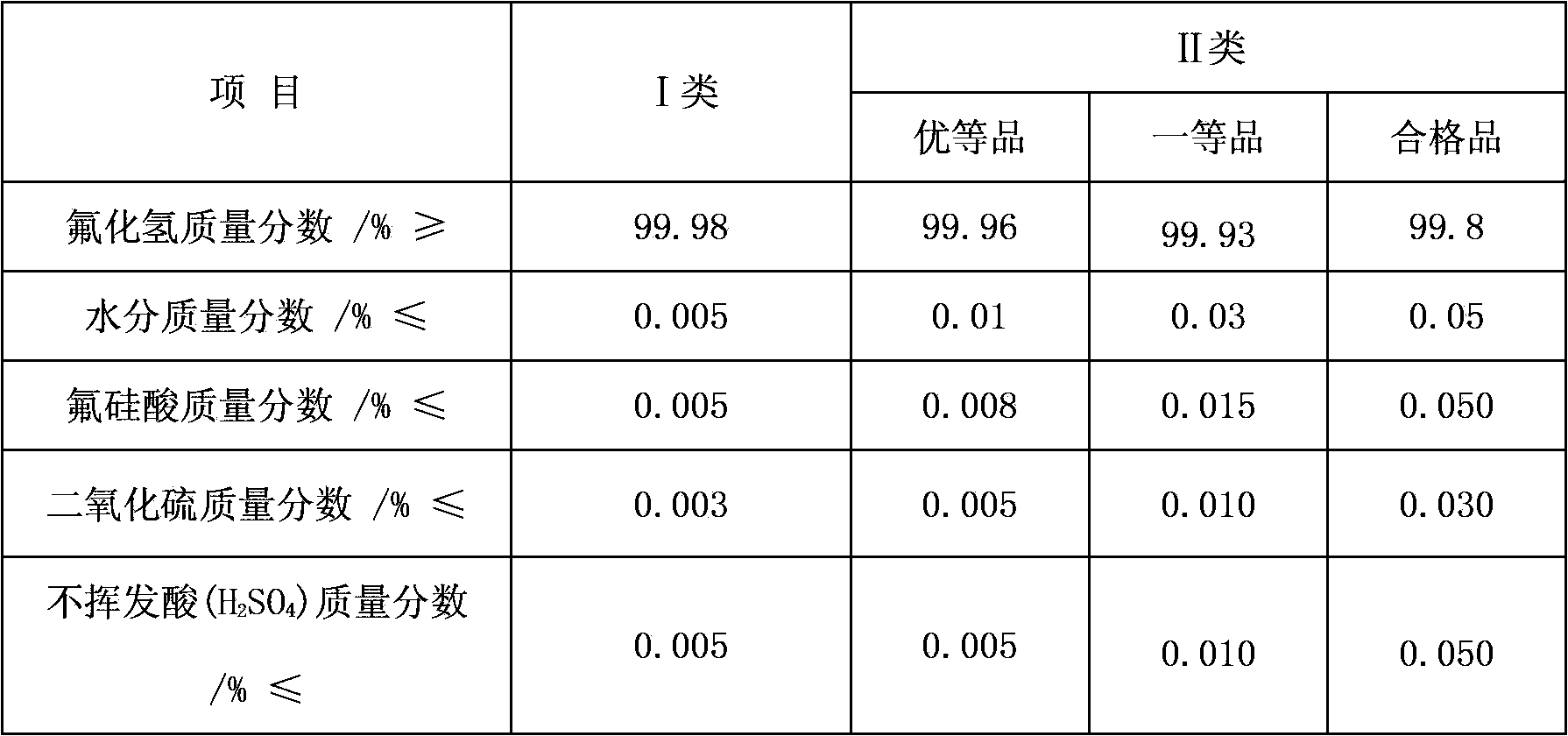
Preparation method for anhydrous hydrogen fluoride
A technology of anhydrous hydrogen fluoride and hydrogen fluoride gas, applied in the direction of fluorine/hydrogen fluoride, etc., can solve problems such as long reaction time, achieve the effects of improving utilization rate, reducing the amount of sulfuric acid, and saving heat energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] a) Pre-reaction
[0069] a-1) Mix concentrated sulfuric acid and fuming sulfuric acid according to the weight ratio of 1:1.43 and send it to the mixed acid heater. The heating time is 7 minutes and the heating temperature is 110°C;
[0070] a-2) Send the fluorspar powder into the powder drying furnace, and use the residual gas heated by the external jacketed converter for heating, the heating time is 75 minutes, and the heating temperature is 175°C;
[0071] a-3) Put the heated mixed sulfuric acid and fluorspar powder into the pre-reactor for mixing according to the weight ratio of 1:1.25 to obtain the mixed material. The mixing temperature is 120°C and the mixing time is 12 minutes. The crude hydrogen fluoride gas produced after mixing accounts for 6% of the total, and enters the converter reactor together with the heated mixed material.
[0072] b) Reaction:
[0073] The heated mixed material is continuously fed into the converter reactor, and the reaction is carrie...
Embodiment 2
[0086] a) Pre-reaction
[0087] a-1) Mix concentrated sulfuric acid and fuming sulfuric acid according to the weight ratio of 1:1.45 and send it to the mixed acid heater, the heating time is 8 minutes, and the heating temperature is 100°C;
[0088] a-2) Send the fluorspar powder into the powder drying furnace, and use the residual gas heated by the external jacketed converter for heating, the heating time is 80 minutes, and the heating temperature is 180°C;
[0089] a-3) Put the heated mixed sulfuric acid and fluorspar powder into the pre-reactor for mixing according to the feeding weight ratio of 1:1.20 to obtain the mixed material. The mixing temperature is 100°C and the mixing time is 15 minutes. The crude hydrogen fluoride gas produced after mixing accounts for 4% of the total, and enters the converter reactor together with the mixed materials.
[0090] b) Reaction:
[0091] In the reaction stage, the temperature in the converter is controlled as front temperature: 550°C...
Embodiment 3
[0100] a) Pre-reaction
[0101] In the pre-reaction stage, except that the weight ratio of concentrated sulfuric acid and oleum is 1:1.40, the heating time is 7 minutes, the heating temperature is 120°C, the heating time of fluorspar powder is 90 minutes, and the heating temperature is 150°C, mixed sulfuric acid and fluorspar powder The weight ratio of the feedstock was 1:1.29, the mixing temperature was 150° C., and the mixing time was 10 minutes. The crude hydrogen fluoride gas generated after mixing accounted for 5% of the total amount, and the rest were the same as in Example 1.
[0102] b) Reaction:
[0103] In the reaction stage, the temperature in the converter is controlled as front temperature: 650°C, middle temperature: 700°C, middle and back temperature: 720°C, back temperature: 750°C, the speed of the converter reactor is 2r / min, and the pressure in the converter is -0.45 KPa, the reaction times is 50 minutes, and the weight ratio of returning material is except t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com