Method for measuring chloride ion in acidity plating solution
A technology of acid plating solution and determination method, which is applied in the field of chemical analysis, can solve the problems of cumbersome methods, expensive prices, and many steps, and achieve the effects of quick operation, simple method, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1 method
[0051] Take 60ml of acidic copper-containing plating solution, add solid sodium hydroxide and stir thoroughly with a glass rod, adjust the pH value of the plating solution to greater than 9, and then filter and remove the precipitate generated in the plating solution. Take 25ml from the filtered plating solution, and adjust the pH value to 9-10 with 10% dilute nitric acid solution.
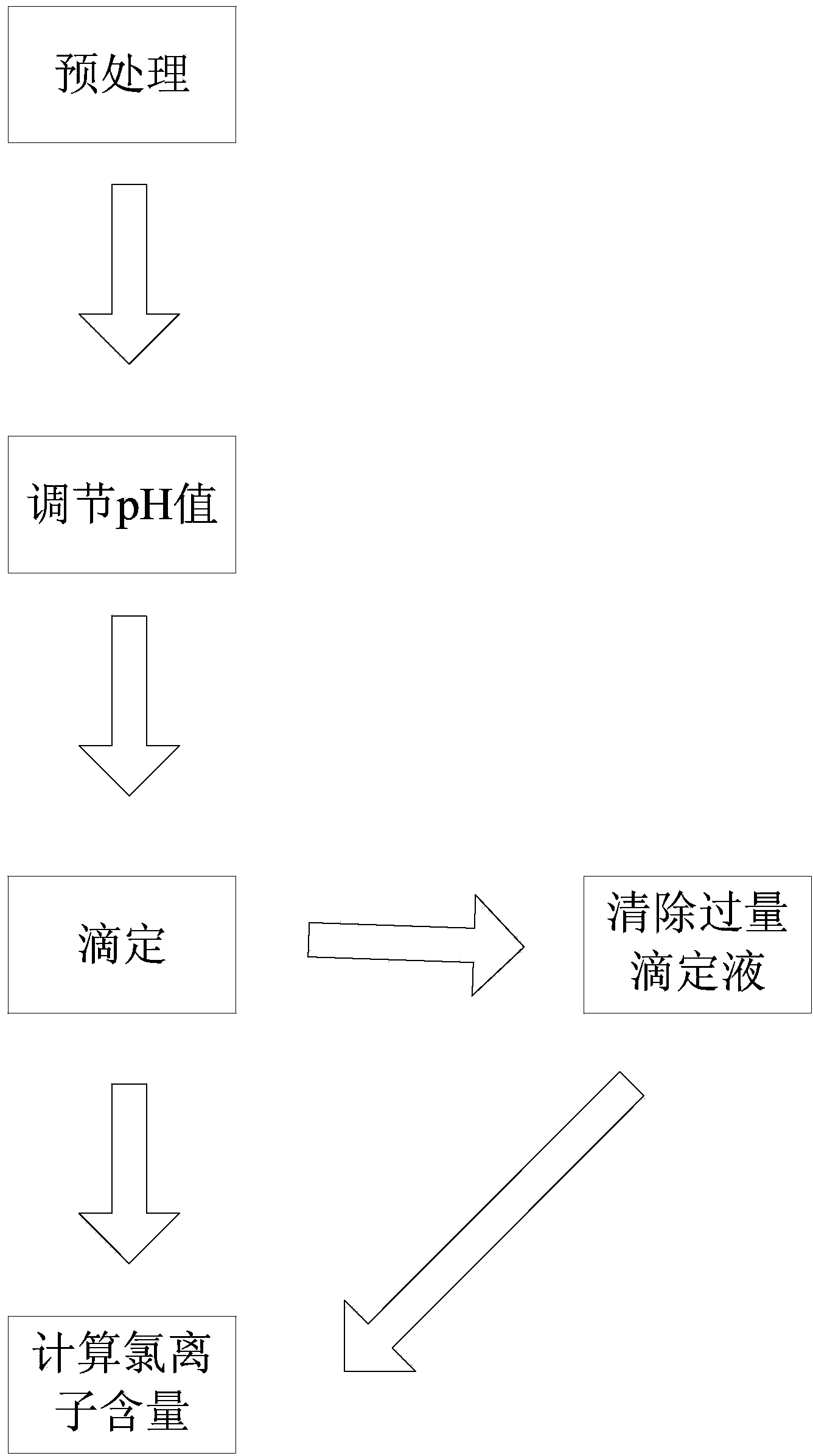
[0052] In the plating solution, add 1ml of 5% potassium chromate, and titrate with 0.05mol / L silver nitrate solution until the chloride ions are completely precipitated, that is, a white precipitate appears until the precipitate turns slightly brick red as the end point. If the silver nitrate solution is used too much, add 2% ferric ammonium sulfate 2ml, and use 0.1mol / L potassium thiocyanate solution to titrate the excess silver nitrate to the red end point. For the specific steps of the determination method, see figure 1 .
[0053] According to the amount of titration solutio...
Embodiment 2
[0059] 1 method
[0060] Take 100ml of acidic nickel-containing plating solution, add solid sodium hydroxide and stir thoroughly with a glass rod, adjust the pH value of the plating solution to greater than 9, and then filter and remove the precipitate generated in the plating solution. Take 25ml from the filtered plating solution, and adjust the pH value to 9-10 with 15% dilute nitric acid solution.
[0061] In the plating solution, add 1ml of 10% potassium chromate, and titrate with 0.1mol / L silver nitrate solution until the chloride ions are completely precipitated, that is, a white precipitate appears until the precipitate turns slightly brick red as the end point. If the silver nitrate solution is used too much, add 2% ferric ammonium sulfate 2ml, and use 0.1mol / L potassium thiocyanate solution to titrate the excess silver nitrate to the red end point. For the specific steps of the determination method, see figure 1 .
[0062] According to the amount of titration soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com