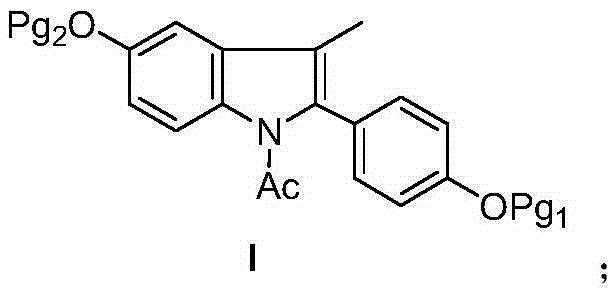
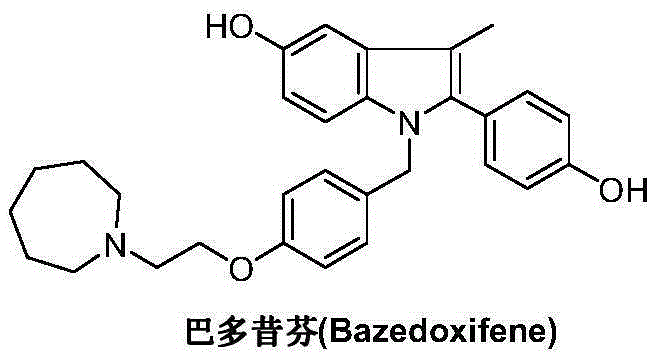
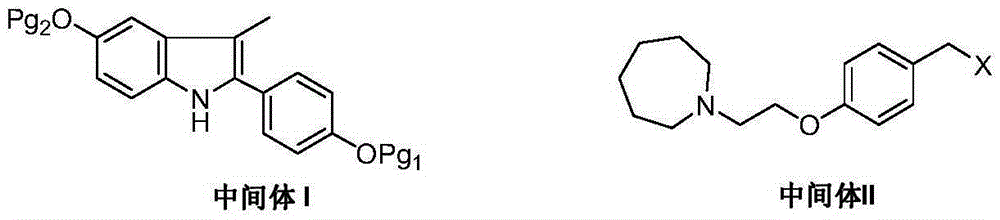Bazedoxifene intermediate and preparation method thereof
An intermediate and oxygen technology, which is applied to the intermediates prepared by the selective estrogen receptor modulator bazedoxifene and its preparation field, can solve the problems of rare raw materials, bromination reaction pollution, and many reaction steps, etc., and achieve The preparation process is quick and convenient, and the product yield and product purity are high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Under an inert atmosphere and anhydrous environment, silver trifluoromethanesulfonate (0.032g, 0.1mmol), bis[dichloro(5-methylcyclopentadienyl) rhodium] (0.015g, 0.025mmol), Copper acetate monohydrate (0.42g, 2.1mmol) and N-(4-methoxyphenyl) acetamide (III) (0.17g, 1mmol) were added to the micro reaction vial, sealed and injected with 1-(4-methanol Oxy-phenyl) propyne (II) (0.15 g, 1 mmol) in 5 mL of tert-butanol was heated to 120° C., stirred for 1 hour, and then cooled to room temperature. TLC detected that the reaction was complete. Filtration, the filter cake was washed with diethyl ether, the filtrate was concentrated, and the residue was subjected to silica gel column chromatography (diethyl ether / petroleum ether=1 / 1) to obtain an off-white solid 2-(4-methoxyphenyl)-3-methyl-5 -Methoxy-1-acetyl-indole (I) 0.26g, yield 84.1%.
Embodiment 2
[0028] Under an inert atmosphere and anhydrous environment, silver trifluoromethanesulfonate (0.032g, 0.1mmol), bis[dichloro(5-methylcyclopentadienyl) rhodium] (0.015g, 0.025mmol), Copper acetate monohydrate (0.42g, 2.1mmol) and N-(4-benzyloxyphenyl) acetamide (III) (0.24g, 1mmol) were added to the micro reaction vial, and 1-(4-benzyl Oxy-phenyl) propyne (II) (0.22 g, 1 mmol) in 5 mL of tert-butanol was heated up to 125° C., stirred for 1 hour, cooled to room temperature, and the reaction was detected by TLC. Filtration, the filter cake was washed with diethyl ether, the filtrate was concentrated, and the residue was subjected to silica gel column chromatography (diethyl ether / petroleum ether=1 / 1) to obtain an off-white solid 2-(4-benzyloxyphenyl)-3-methyl-5 - Benzyloxy-1-acetyl-indole (I) 0.41 g, yield 89.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com