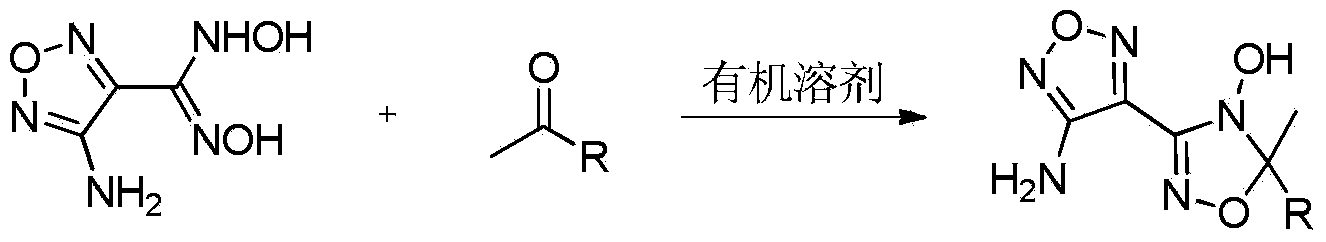
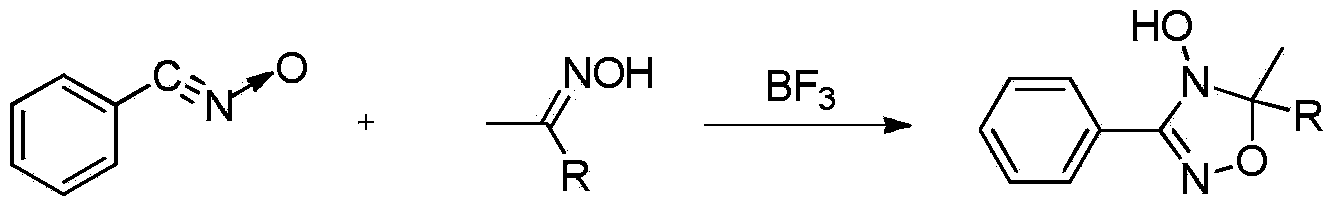Synthetic method of 3-(3-aminofurazan-4-yl)-5-methyl-5-substitute-4-hydroxy-4H,5H-1,2,4-oxadiazole compound
A technology of aminofurazan and synthesis method, applied in the direction of organic chemistry, etc., can solve the problem of low reaction yield and achieve the effect of high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of 3-(3-aminofurazan-4-yl)-5,5-dimethyl-4-hydroxy-4H,5H-1,2,4-oxadiazole compound (Ⅰa)
[0020]
[0021] At a temperature of 20°C, add 0.477g (3mmol) of 3-amino-4-gemdioximofurazan and 6mL of ethanol into a round-bottomed flask with a suitable size magnet, stir and dissolve and add 0.87g (15mmol) ) acetone, and reacted for 2 hours at a temperature of 0° C. under stirring. After the reaction was completed, the ethanol was evaporated, and the resulting product was separated by column chromatography (the eluent was petroleum ether and ethyl acetate, and its volume ratio was 3:1) to obtain 3-(3-aminofurazan-4-yl)- 5,5-Dimethyl-4-hydroxy-4H,5H-1,2,4-oxadiazole, yield 98%.
[0022] Structure Identification:
[0023] Infrared Spectrum: IR(KBr,cm -1 )ν: 3467, 3339, 1636, 1604, 1523, 1375, 1103, 949, 800;
[0024] NMR: 1 H NMR (DMSO-d 6 ,500MHz), δ:9.81(s,1H,OH),6.33(s,2H,NH 2 ),1.49(s,6H,CH 3 );
[0025] 13 C NMR (DMSO-d 6 ,125MHz),δ:155.29,150.93,136....
Embodiment 2
[0031] Preparation of 3-(3-aminofurazan-4-yl)-5,5-dimethyl-4-hydroxy-4H,5H-1,2,4-oxadiazole compound (Ⅰa)
[0032]
[0033] At a temperature of 25°C, add 0.477g (3mmol) of 3-amino-4-gemdioximefuran and 3mL of tetrahydrofuran into a round-bottomed flask with a suitable size magnet, stir to dissolve and add 0.348g (6mmol) ) acetone, and reacted for 2 hours at a temperature of 20° C. under stirring. After the reaction was completed, tetrahydrofuran was distilled off, and the obtained product was separated by column chromatography (eluent was petroleum ether and ethyl acetate, the volume ratio was 3:1), and the target product Ia was obtained with a yield of 36%.
[0034] Structure Identification:
[0035] Infrared Spectrum: IR(KBr,cm -1 )ν: 3467, 3339, 1636, 1604, 1523, 1375, 1103, 949, 800;
[0036] NMR: 1 H NMR (DMSO-d 6 ,500MHz), δ:9.81(s,1H,OH),6.33(s,2H,NH 2 ),1.49(s,6H,CH 3 );
[0037] 13 C NMR (DMSO-d 6 ,125MHz),δ:155.29,150.93,136.27,102.82,21.78;
[0038] El...
Embodiment 3
[0043] Preparation of 3-(3-aminofurazan-4-yl)-5-methyl-5-ethyl-4-hydroxy-4H,5H-1,2,4-oxadiazole compound (Ⅰb)
[0044]
[0045] At a temperature of 25°C, add 0.477g (3mmol) of 3-amino-4-gemdioximefurazan and 6mL of isopropanol into a round-bottomed flask with a suitable size magnet, stir to dissolve and add 0.540g (7.5 mmol) methyl ethyl ketone, and reacted at a temperature of 40° C. under stirring for 3 hours. After the reaction is completed, the isopropanol is evaporated, and the resulting product is separated by column chromatography (eluent is petroleum ether: ethyl acetate=3:1, volume ratio), and the target product 3-(3-aminofurazan-4- Base)-5-methyl-5-ethyl-4-hydroxyl-4H,5H-1,2,4-oxadiazole Ib, yield 72%.
[0046] The structural confirmation data of this product are as follows:
[0047] Infrared Spectrum: IR(KBr,cm -1 )ν: 3469, 3341, 1636, 1604, 1528, 1376, 1124, 958, 821;
[0048] NMR: 1 H NMR (DMSO-d 6 ,500MHz), δ:9.79(s,1H,OH),6.32(s,2H,NH 2 ),1.71-1.85(m,2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com