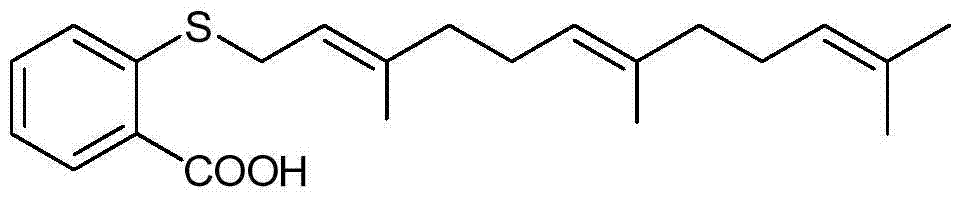
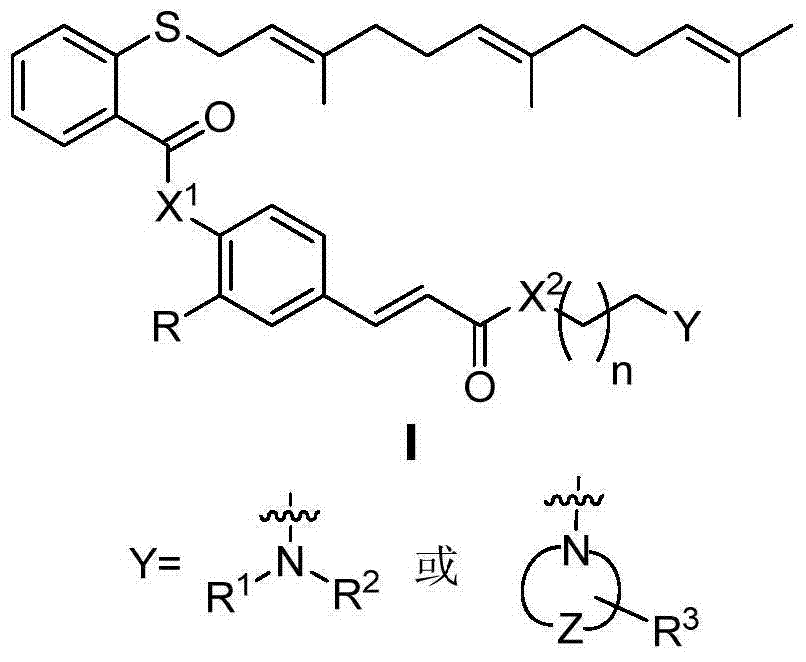
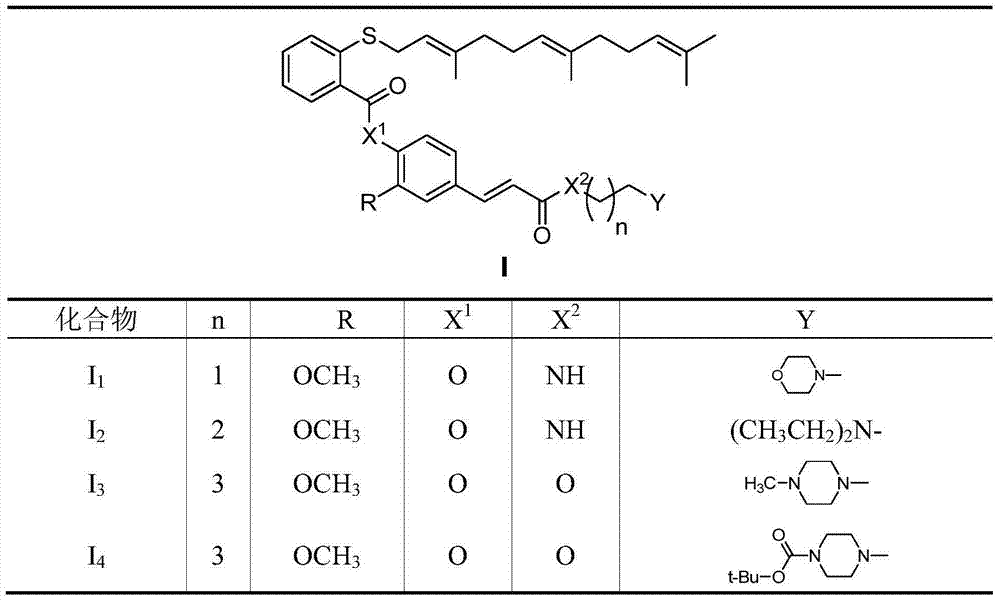
Phenyl acrylic acid farnesyl thiosalicylic acid (FTA) derivative as well as preparation method and application
A technology of farnesyl thiosalicylic acid and farnesyl thiosalicylate, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1 2-methoxy-4-((E)-3-(2-morpholinoethylamino)-3-oxapropenyl)phenyl-farnesylthiosalicylate (I 1 ) preparation
[0088] Preparation of (E)-3-(4-hydroxy-3-methoxyphenyl)-N-(2-morpholinoethyl)acrylamide (5a)
[0089] 2-Morpholinoethylamine (2.03 g, 15.5 mmol) and triethylamine (4.70 g, 46.5 mmol) were dissolved in 20 mL of anhydrous CH 2 Cl 2 In ice bath, slowly dropwise add acetyl ferulic acid chloride (6a, 1.31g, 5.16mmol) in CH 2 Cl 2 Solution 20mL, remove the ice bath after dripping, continue to stir at room temperature, spot the plate until the reaction is complete. Concentrate the reaction solution, dissolve the concentrate in 10 mL of methanol, add 1N NaOH solution, continue to stir at room temperature for 2 hours, adjust the pH to neutral, concentrate to make sand, and pass through the column (PE:AE=1:1~1:4). 1.17 g of a colorless transparent viscous liquid was obtained, with a yield of 74%.
[0090] 2-Methoxy-4-((E)-3-(2-morpholinoethylamino)-3-oxapro...
Embodiment 2
[0093] Example 2 2-methoxy-4-((E)-3-(2-(diethylamino)propylamino)-3-oxapropenyl)phenyl-farnesyl thiosalicylate (I 2 ) preparation
[0094] Preparation of (E)-N-(2-(diethylamino)propyl)-3-(4-hydroxyl-3-methoxyphenyl)acrylamide (5b)
[0095] Referring to the synthesis method of (5a), acetyl ferulic acid chloride (6a, 1.31g, 5.16mmol) was reacted with diethylaminopropylamine (2.02g, 15.5mmol) to obtain 1.06g of colorless transparent viscous liquid (67 %).
[0096] 2-Methoxy-4-((E)-3-(2-(diethylamino)propylamino)-3-oxapropenyl)phenylfarnesylthiosalicylate (I 2 ) preparation
[0097] Refer to (I 1 ) synthesis method, from FTA (0.32g, 0.49mmol), 5b (0.16g, 0.51mmol) to obtain a colorless transparent viscous liquid 0.22g, yield 71%.
[0098] 1 H NMR (CDCl 3 ,300MHz):δ8.25(d,1H,J=7.8Hz,Ar-H),7.66(m,1H,Ar-H),7.51(m,2H,Ar-H,COCH=C H ),7.36(m,2H,Ar-H),7.23(m,2H,Ar-H),6.39(d,1H,J=16.2Hz,COCH),5.35(m,1H,SCH 2 C H ),5.09(m,2H,2×CH 2 C H =CCH 3 ),3.59(m,5H,SCH 2 ,OCH 3 ),2.50...
Embodiment 3
[0099] Example 3 2-Methoxy-4-((E)-3-(4-methylpiperazin-1-yl)butoxy)-3-oxapropenyl)phenyl farnesylthio water Salylate (I 3 ) preparation
[0100] Preparation of (E)-4-bromobutyl-3-(3-acetoxy-4-hydroxyphenyl)acrylate (2a)
[0101] Ferulic acid (1a, 5g, 25.8mmol) was dissolved in 50mL of acetone, 1,4-dibromobutane (21.6g, 100mmol), 10mL of Et 3 N, heated at an external temperature of 50°C for 4h. After cooling to room temperature, a large amount of white solid precipitated, filtered, the filtrate was concentrated, and column chromatography [ethyl acetate: petroleum ether (60-90°C) = 1:4 (V:V)] was eluted to obtain a light yellow needle-like solid 6.85g (77%), mp 96-98°C.
[0102] Preparation of 2-methyl-4-((E)-3-(4-bromobutoxy)-3-oxapropenyl)-phenyl-farnesylthiosalicylate (3a)
[0103] Dissolve FTA (2.4 g, 6.70 mmol), 2a (2.31 g, 7.04 mmol), DMAP (0.70 g, 5.70 mmol) in 30 mL of anhydrous CH 2 Cl 2 , stirring, slowly drop DCC (1.66g, 8.04mmol) in CH under ice bath 2 Cl 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com